kentchemistry.com. The fluorine would have a '+' partial charge, and the boron a '-' partial charge, this is inconsistent with the electronegativities of fluorine and boron. So first we look at how many electrons we have so for valence electrons chlorine has 7 and each oxygen has 6. The electron geometry of ClO2- is tetrahedral. Whereas these two electrons lost by Mg are gained by oxygen to complete its stable octet. (b) a Lewis structure in which all the formal charges are zero. Ocean currents that move up and down are called surface currents. WebPhosphoryl chloride, POCl3, has the skeleton structure Write (a) a Lewis structure for POCl3 following the octet rule. Thus a reaction occurs to do so and during that reaction as the stability of the atom increases it will release energy in the form of heat or light. An example of this would be Nitrogen (II) Oxide (NO ,refer to figure one). Both sodium and chlorine share their electron and complete their octet by forming Sodium Chloride (NaCl) as shown below with the help of Lewis dot structure: The octet rule helps us predict the chemical behaviour of the elements. Sulphur hexafluoride (SF, ) and phosphorus pentachloride are 2 examples (PCl, ) in a big way. Published By Vishal Goyal | Last updated: December 30, 2022, Home > Chemistry > ClO2- lewis structure and its molecular geometry. Hence, the valence electron for chlorine is 7 and for oxygen, it is 6. Definitely ClO2- is polar in nature as it lacks symmetry because it has a molecular geometry of bent, which means dipole generated along with Cl-O bond unable to canceled out each other giving some dipole moment in the molecule. Here, it is important to know that the valence electron present as the (-) sign on the oxygen atom is responsible for the bond formation because it is unstable and needs more valence electrons to achieve a stable configuration. },{ Both sodium and chlorine share their electrons and complete their octet by forming Sodium Chloride (NaCl). "@type": "Question", (The octet rule need not be followed.) All the atoms have eight electrons in their outer shell so they follow the octet rule. The conjugate acid of chlorite ion is chlorous acid. Hence, to attain stability the oxygen molecule reacts with another oxygen molecule forming a double bond and sharing in total 4 electrons amongst themselves. Calculate the formal charges in this structure. n=3) and beyond. "text": "Whenever the d-orbitals and beyond to it, participate in bonding with other atoms then an expanded octet is produced. If one was to make a Lewis structure for BH3 following the basic strategies for drawing Lewis structures, one would probably come up with this structure (Figure 3.8.3): The problem with this structure is that boron has an incomplete octet; it only has six electrons around it. It is used by healthcare professionals during various dental applications. Both sodium and chlorine share their electrons and complete their octet by forming Sodium Chloride (NaCl). Does ClO2 Follow The Octet Rule Get the answers you need, now! YouTube. As an opposite charge is being applied electrostatically between the ions, an ionic bond is formed. Thus, the atoms of different elements react with each other to get the most stable state. Examples of stable odd-electron molecules are The lone electron is called an unpaired electron. Here each carbon atom requires two electrons to complete its octet. Thus during this reaction, the Mg and O are bonded to each other by an ionic bond. Elements like hydrogen, lithium, helium do not obey the octet rule. (The octet rule need not be followed.) 5. WebWith an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. The octet rule states that elements will gain or lose electrons in order to have a full outer shell of eight electrons. So, we put the 6 valence electron on each oxygen, as oxygen already shares two-electron with the help of a single bond. One has a unit negative charge on it and the other three are neutral. The octet rule states that an atom tends to have eight electrons in its outermost valence shell by forming covalent bonds through gaining or losing electrons from its outermost shell. tejvirsinghu4084 tejvirsinghu4084 13.04.2020 Chemistry Secondary School answered Does ClO2 Follow The Octet Rule See answers Advertisement Advertisement saloniaggarwal907 saloniaggarwal907 Answer: yes it follows the rule. Despite the cases for expanded octets, as mentioned for incomplete octets, it is important to keep in mind that, in general, the octet rule applies. In this ion, the chlorine atom does follow the octet rule, unlike ClO 3, or ClO 4. The bond angle of ClO2- is less than 109 due to the presence of two lone pairs on chlorine atoms as these lone pairs repel each other and that pushes bonded atoms closer together, hence causes the lower bond angle. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. Therefore the above structure is unstable because there exists another Lewis structure where the formal distribution of some participating atoms is zero. Solve any question of Chemical Bonding and Molecular Structure with:-Patterns of problems > This arises from the fact that chlorine dioxide is an unstable molecule and mainly exists as ClO2- during bond formation. 1. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. Notify me of follow-up comments by email. The octet rule is clearly violated in this case. Chlorine dioxide is a polar molecule because it is a strong anion and exists in the ionic form. The lone pair presence on chlorine causes an unsymmetrical charge distribution in the molecule, so the induced charges on the Cl-O bond do not cancel each other completely. WebAlthough they are few, some stable compounds have an odd number of electrons in their valence shells. Identify the atom and explain the deviation from the octet rule: (a) BeCl2; (b) ClO2. Examples of stable odd-electron molecules are The structure with the formal charge close to zero or zero is the best and stable lewis structure. Hence, stable molecules, ions and atoms are expected to contain atoms that obey the octet rule. This does not mean that the octet rule is uselessquite the contrary. If all of the phosphorus-chlorine particularly links during a PCl, will make a case for the creation of 5 bonds by phosphorus molecules, which essentially is quite significant. 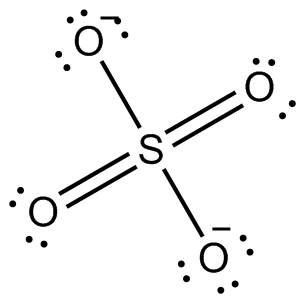 Valence electrons mean the total number of electrons present in the outermost shell of an element that can participate in the bond formation. However, it is hard to imagine that one rule could be followed by all molecules. This structure is supported by the fact that the experimentally determined bond length of the boron to fluorine bonds in BF3 is less than what would be typical for a single bond (see Bond Order and Lengths). This results in nitrogen having a formal charge of +1. Copyright 2023 - topblogtenz.com. Molecular geometry is the three-dimensional arrangement of atoms in a molecule. 5. Lewis Dot Structure of ClO2- (Chlorite Ion) Watch later. Formal charge = (valence electrons lone pair electrons 1/2bonded pair electrons), Bonded pair electrons around chlorine = 4, F.C. Advertisement.
Valence electrons mean the total number of electrons present in the outermost shell of an element that can participate in the bond formation. However, it is hard to imagine that one rule could be followed by all molecules. This structure is supported by the fact that the experimentally determined bond length of the boron to fluorine bonds in BF3 is less than what would be typical for a single bond (see Bond Order and Lengths). This results in nitrogen having a formal charge of +1. Copyright 2023 - topblogtenz.com. Molecular geometry is the three-dimensional arrangement of atoms in a molecule. 5. Lewis Dot Structure of ClO2- (Chlorite Ion) Watch later. Formal charge = (valence electrons lone pair electrons 1/2bonded pair electrons), Bonded pair electrons around chlorine = 4, F.C. Advertisement.  However, if we add the eleventh electron to nitrogen (because we want the molecule to have the lowest total formal charge), it will bring both the nitrogen and the molecule's overall charges to zero, the most ideal formal charge situation. Due to this, the valence electrons in chlorine dioxide or chlorite are 20. The electron geometry for ClO2- is tetrahedral. The Lewis electron dot diagram for NO is as follows: Although the O atom has an octet of electrons, the N atom has only seven electrons in its valence shell. Radicals are found as both reactants and products, but generally react to form more stable molecules as soon as they can. WebIn this problem, we're looking at c o 2 and told chlorine will have an expanded octet. Examples of stable odd-electron molecules are \(\ce{NO}\), \(\ce{NO2}\), and \(\ce{ClO2}\). Save my name, email, and website in this browser for the next time I comment. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. Both sodium and chlorine share their electrons and complete their octet by forming Sodium Chloride (NaCl). Chlorine contains seven electrons in its outermost shell and requires only one electron to complete its octet whereas sodium contains one electron in its outermost shell. Oxygen therefore has a formal charge of 0. Interestingly, odd Number of Valence Electrons will result in the molecule being paramagnetic. The orbital diagram for the valence shell of phosphorous is: Hence, the third period elements occasionally exceed the octet rule by using their empty d orbitals to accommodate additional electrons. It might confuse many people as ClO2 comprises 19 valence electrons only. They can only lose or gain one electron to become stable due to which they follow the octet rule. So, for a hybridization number of four, we get the Sp3 hybridization on the chlorine atom in the ClO2- molecule. These electrons are less stable and do not obey the octet rule. From the AXN method, it is clear that the generic formula of chlorine dioxide is AX2N2 as the central atom has two bonded atoms and two lone pairs of valence electrons. Which of the following are found in our solar system? This is the same amount as the number of valence electrons they would have on their own, so they both have a formal charge of zero. Connect outer atoms to central atom with a single bond. However, it is hard to imagine that one rule could be followed by all molecules. Chlorite is used in the paper, pulp, and bleaching of textiles. "name": "Why does the chlorine in ClO2- lewis structure violate the octet rule? Hence, all these factors lead to ClO2- a polar molecule in nature. Question: Which one of the following compounds does not follow the octet rule? The sp3d interbreeding in PCl5 will make a case for the creation of 5 bonds by phosphorus molecules, which essentially is quite significant. Noble gasses are said to be highly stable elements. For instance, in ClO, chlorine has seven valence electrons and oxygen has six electrons. It is clear from the chemical formula that chlorine dioxide or chlorite is a strong oxidizing agent. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Add a multiple bond (double bond) to see if central atom can achieve an octet: In this structure with a double bond the fluorine atom is sharing extra electrons with the boron. Verified by Toppr. Your email address will not be published. Examples of stable odd-electron molecules are NO, NO 2, and ClO 2. The number of and values of the formal charges on this structure (-1 and 0 (difference of 1) in Figure 3.8.12, as opposed to +2 and -1 (difference of 3) in Figure 3.8.12) is significantly lower than on the structure that follows the octet rule, and as such an expanded octet is plausible, and even preferred to a normal octet, in this case. Chlorine belongs to the third group in the periodic table. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Now we associate the sp3d hybrid, generally created by the hybridization of one orbital 3p, and one d sort of orbital in a subtle way. The Xe atom has an expanded valence shell with more than eight electrons around it. How to tell if a molecule is polar or nonpolar? 4. Similarly, chlorine shares 1 electron with oxygen ion with a negative charge on it, forming a single bond with it. So, from chlorine and oxygen, chlorine(3.16) is less electronegative than oxygen(3.44). The octet corresponds to an electronic configuration of s2p6 because the octet rule only involves the s and p electrons. WebThis problem has been solved! The lewis dot structure of ClO2- contains 7 lone pairs and 3 bonded pairs. s-block and p-block elements obey the octet rule except for hydrogen, helium, and lithium. The hybridization can be studied in the detail with the help of Valence Bond Theory (VBT). It makes sense why Be does not follow the octet rule because it only has 4 Magnesium reacts with oxygen to form magnesium oxide. H2CO lewis structure, molecular geometry, polarity,, CHCl3 lewis structure, molecular geometry, polarity,, N2H4 lewis structure, molecular geometry, polarity,, AX3E Molecular geometry, Hybridization, Bond angle, Polarity, AX2E3 Molecular geometry, Hybridization, Bond angle,, AX4E2 Molecular geometry, Bond angle, Hybridization,, AX2E2 Molecular geometry, Bond angle, Hybridization,, AX2E Molecular geometry, Hybridization, Bond angle, Polarity, N2H2 Lewis structure, molecular geometry, hybridization,, C3H6 Lewis structure, Hybridization, Molecular geometry,. Calculate the formal charges in this structure. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. The Mg loses two electrons and forms a stable octet with 12 protons and 10 electrons in the L shell. However, this structure contradicts one of the major rules of formal charges: Negative formal charges are supposed to be found on the more electronegative atom(s) in a bond, but in the structure depicted in Figure 3.8.5, a positive formal charge is found on fluorine, which not only is the most electronegative element in the structure, but the most electronegative element in the entire periodic table (\(\chi=4.0\)). Webdoes clo2 follow the octet rule. Hypervalent compounds are formed by some main cluster elements. Unit 3: Chemical Bonding I - Lewis Theory, { "3.1:_Chemical_Bonds_Lewis_Symbols_and_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
However, if we add the eleventh electron to nitrogen (because we want the molecule to have the lowest total formal charge), it will bring both the nitrogen and the molecule's overall charges to zero, the most ideal formal charge situation. Due to this, the valence electrons in chlorine dioxide or chlorite are 20. The electron geometry for ClO2- is tetrahedral. The Lewis electron dot diagram for NO is as follows: Although the O atom has an octet of electrons, the N atom has only seven electrons in its valence shell. Radicals are found as both reactants and products, but generally react to form more stable molecules as soon as they can. WebIn this problem, we're looking at c o 2 and told chlorine will have an expanded octet. Examples of stable odd-electron molecules are \(\ce{NO}\), \(\ce{NO2}\), and \(\ce{ClO2}\). Save my name, email, and website in this browser for the next time I comment. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. Both sodium and chlorine share their electrons and complete their octet by forming Sodium Chloride (NaCl). Chlorine contains seven electrons in its outermost shell and requires only one electron to complete its octet whereas sodium contains one electron in its outermost shell. Oxygen therefore has a formal charge of 0. Interestingly, odd Number of Valence Electrons will result in the molecule being paramagnetic. The orbital diagram for the valence shell of phosphorous is: Hence, the third period elements occasionally exceed the octet rule by using their empty d orbitals to accommodate additional electrons. It might confuse many people as ClO2 comprises 19 valence electrons only. They can only lose or gain one electron to become stable due to which they follow the octet rule. So, for a hybridization number of four, we get the Sp3 hybridization on the chlorine atom in the ClO2- molecule. These electrons are less stable and do not obey the octet rule. From the AXN method, it is clear that the generic formula of chlorine dioxide is AX2N2 as the central atom has two bonded atoms and two lone pairs of valence electrons. Which of the following are found in our solar system? This is the same amount as the number of valence electrons they would have on their own, so they both have a formal charge of zero. Connect outer atoms to central atom with a single bond. However, it is hard to imagine that one rule could be followed by all molecules. Chlorite is used in the paper, pulp, and bleaching of textiles. "name": "Why does the chlorine in ClO2- lewis structure violate the octet rule? Hence, all these factors lead to ClO2- a polar molecule in nature. Question: Which one of the following compounds does not follow the octet rule? The sp3d interbreeding in PCl5 will make a case for the creation of 5 bonds by phosphorus molecules, which essentially is quite significant. Noble gasses are said to be highly stable elements. For instance, in ClO, chlorine has seven valence electrons and oxygen has six electrons. It is clear from the chemical formula that chlorine dioxide or chlorite is a strong oxidizing agent. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Add a multiple bond (double bond) to see if central atom can achieve an octet: In this structure with a double bond the fluorine atom is sharing extra electrons with the boron. Verified by Toppr. Your email address will not be published. Examples of stable odd-electron molecules are NO, NO 2, and ClO 2. The number of and values of the formal charges on this structure (-1 and 0 (difference of 1) in Figure 3.8.12, as opposed to +2 and -1 (difference of 3) in Figure 3.8.12) is significantly lower than on the structure that follows the octet rule, and as such an expanded octet is plausible, and even preferred to a normal octet, in this case. Chlorine belongs to the third group in the periodic table. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Now we associate the sp3d hybrid, generally created by the hybridization of one orbital 3p, and one d sort of orbital in a subtle way. The Xe atom has an expanded valence shell with more than eight electrons around it. How to tell if a molecule is polar or nonpolar? 4. Similarly, chlorine shares 1 electron with oxygen ion with a negative charge on it, forming a single bond with it. So, from chlorine and oxygen, chlorine(3.16) is less electronegative than oxygen(3.44). The octet corresponds to an electronic configuration of s2p6 because the octet rule only involves the s and p electrons. WebThis problem has been solved! The lewis dot structure of ClO2- contains 7 lone pairs and 3 bonded pairs. s-block and p-block elements obey the octet rule except for hydrogen, helium, and lithium. The hybridization can be studied in the detail with the help of Valence Bond Theory (VBT). It makes sense why Be does not follow the octet rule because it only has 4 Magnesium reacts with oxygen to form magnesium oxide. H2CO lewis structure, molecular geometry, polarity,, CHCl3 lewis structure, molecular geometry, polarity,, N2H4 lewis structure, molecular geometry, polarity,, AX3E Molecular geometry, Hybridization, Bond angle, Polarity, AX2E3 Molecular geometry, Hybridization, Bond angle,, AX4E2 Molecular geometry, Bond angle, Hybridization,, AX2E2 Molecular geometry, Bond angle, Hybridization,, AX2E Molecular geometry, Hybridization, Bond angle, Polarity, N2H2 Lewis structure, molecular geometry, hybridization,, C3H6 Lewis structure, Hybridization, Molecular geometry,. Calculate the formal charges in this structure. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. The Mg loses two electrons and forms a stable octet with 12 protons and 10 electrons in the L shell. However, this structure contradicts one of the major rules of formal charges: Negative formal charges are supposed to be found on the more electronegative atom(s) in a bond, but in the structure depicted in Figure 3.8.5, a positive formal charge is found on fluorine, which not only is the most electronegative element in the structure, but the most electronegative element in the entire periodic table (\(\chi=4.0\)). Webdoes clo2 follow the octet rule. Hypervalent compounds are formed by some main cluster elements. Unit 3: Chemical Bonding I - Lewis Theory, { "3.1:_Chemical_Bonds_Lewis_Symbols_and_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.2:_Ionic_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.3:_Covalent_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.4:_Bond_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.5:_Drawing_Lewis_Structures" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.6:_Resonance_Structures" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.7:_Electron_Pushing_Arrows" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.8:_Exceptions_to_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.E:_Exercises" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", Prerequisite_Knowledge : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_0:_Foundations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_1:_Quantum_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_2._Periodic_Properties_of_the_Elements" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_3:_Chemical_Bonding_I_-_Lewis_Theory" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_4:_Chemical_Bonding_II_-_Advanced_Bonding_Theories" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_5:_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_6:_Introduction_to_Organic_Nomenclature" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, [ "article:topic", "showtoc:no", "license:ccbyncsa", "licenseversion:40" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FMount_Royal_University%2FChem_1201%2FUnit_3%253A_Chemical_Bonding_I_-_Lewis_Theory%2F3.8%253A_Exceptions_to_the_Octet_Rule, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), Exception 1: Species with Odd Numbers of Electrons, http://www.saskschools.ca/curr_content/chem20/covmolec/exceptns.html, http://www.youtube.com/watch?v=KEQw9uQ8fUU, status page at https://status.libretexts.org, To understand three ways that molecules and ions violate the Octet Rule, When there are an odd number of valence electrons, When there are too many valence electrons. In each of these compounds, an atom violates the octet rule. The Octet Rule for this molecule is fulfilled in the above example, however that is with 10 valence electrons. Techiescientist is a Science Blog for students, parents, and teachers. One single bond is formed between chlorine and oxygen atoms and a double bond is formed between chlorine and another oxygen atom. [Exception to the octet rule]. There are even more occasions where the octet rule does not give the most correct depiction of a molecule or ion. Lewis Structure of Chlorine Dioxide (ClO2-), H2O2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. Author: Published in: merseyrail incident today abril 5, 2023 Categories: netball defence drills pdf in NO and NO2 molecule less than 8 electron . The magnesium has two electrons in its outermost orbit i.e., M shell and oxygen needs two electrons to form a stable octet. The Lewis electron dot diagram for NO is as follows: Nitrogen and oxygen share four electrons between them. WebAlthough they are few, some stable compounds have an odd number of electrons in their valence shells. It is used during food processing to preserve it for a longer duration. Also, the presence of a negative charge over the atoms makes the Cl-O bonds polar in nature. We have left with 16 valence electrons more. 3. ClO2 molecule expend you octate . Whereas in NH3 and H2O there are 1 and 2 lone pair of electrons respectively on central atom. In Figure 3.8.1, it has two lone pair electrons and it participates in two bonds (a double bond) with oxygen.  The species that do not obey the octet rule are; ClO, ClO2^-, ClO3^-, ClO4^-. The rule states that the difference between the maximum negative and positive valence of an element is 8. Sulfur, phosphorous, and silicon are some other examples that can expand their octet and hold electrons more than 8."
The species that do not obey the octet rule are; ClO, ClO2^-, ClO3^-, ClO4^-. The rule states that the difference between the maximum negative and positive valence of an element is 8. Sulfur, phosphorous, and silicon are some other examples that can expand their octet and hold electrons more than 8." /Octet-Rule-58e284343df78c5162ec2284.jpg) An example of a stable molecule with an odd number of valence electrons would be nitrogen monoxide. 70 More Lewis Dot Structures. So, chlorine and both neutral oxygen share 2 electrons to get a stable octet and form a double bond. 3. Check the stability with the help of a formal charge concept, A formal charge is a charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms.. In this ion, the chlorine atom does follow the octet rule, unlike ClO 3, or ClO 4. Odd-electron molecules are the first violation to the octet rule. Thus, a pair of dots represent the bond between the chemical symbols of the atom. ClO2- lewis structure comprises two oxygen (O) atoms and one chlorine (Cl)atom. Let's take a look at one such hydride, BH3 (Borane). We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. In CH4 and PCl5 there are no lone pair of electrons on central atom. Identify the atom and explain the deviation from the octet rule: (a) BeCl2; (b) ClO2. Sulfur, phosphorous, and silicon are some other examples that can expand their octet and hold electrons more than 8. The octet rule states that when an element loses, gains, or shares its outermost electrons to complete their octet state with a set of eight electrons then it Is said that they are following the octet rule. For the sigma bond, head-on overlapping takes place whereas, for the pi bond, lateral overlapping takes place. It is shown below with the help of Lewis dot structure: NCERT Class 11 Chemistry Part 1. It is a classic example of chemistry being full of exceptions! As with many rules, there are exceptions, or violations. Required fields are marked *. Chlorine contains seven electrons in its outermost shell and requires only one electron to complete its octet whereas sodium contains one electron in its outermost shell. As a result, chlorine becomes the central atom. WebIn order to do that we will calculate the total number of valence electrons in each of the following and then write the Lewis structure. The octet rule states that atoms must have eight electrons in their outermost shell in order to attain stability. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/, Your email address will not be published. It is 20 as chlorine has 7 valence electrons and oxygen has 6 valence electrons. 11 Uses of Platinum Laboratory, Commercial, and Miscellaneous, CH3Br Lewis Structure, Geometry, Hybridization, and Polarity. Here +1 formal charge of the chlorine atom cancels out the -1 formal charge of one of the oxygen atoms. }, Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. In this ion, the chlorine atom does follow the octet rule, unlike ClO 3, or ClO 4. So, start putting the remaining valence electrons on the outer atom(oxygen) and complete their octet first. The explanation for the same lies in the formal charge distribution that compels the formation of a double bond and a single bond. This is the same amount of electrons as the number of valence electrons that oxygen atoms have on their own, and as such both of these oxygen atoms have a formal charge of zero. NCERT Class 11 Chemistry Part 1. Again, chlorine is able to expand its octet (contain more than eight valence electrons). Solution. Identify the atom and explain the deviation from the octet rule: (a) BeCl2; (b) ClO2. clo clo clo2 clo3 clo4? A bond angle is the geometrical angle between two adjacent bonds. If you need more information about formal charges, see Lewis Structures. True or false? Although the electronegativity difference between oxygen and chlorine atoms is less than 0.4, chlorine dioxide is a polar molecule which is due to the fact that it is a strong anion. The Lewis electron dot diagram for NO is as follows: Nitrogen and oxygen share four electrons between them. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. Now we need to calculate the formal charge distribution on chlorine dioxide molecule: Formal Charge = Valence Electrons Non-Bonding Electrons Bonding Electrons.
An example of a stable molecule with an odd number of valence electrons would be nitrogen monoxide. 70 More Lewis Dot Structures. So, chlorine and both neutral oxygen share 2 electrons to get a stable octet and form a double bond. 3. Check the stability with the help of a formal charge concept, A formal charge is a charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms.. In this ion, the chlorine atom does follow the octet rule, unlike ClO 3, or ClO 4. Odd-electron molecules are the first violation to the octet rule. Thus, a pair of dots represent the bond between the chemical symbols of the atom. ClO2- lewis structure comprises two oxygen (O) atoms and one chlorine (Cl)atom. Let's take a look at one such hydride, BH3 (Borane). We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. In CH4 and PCl5 there are no lone pair of electrons on central atom. Identify the atom and explain the deviation from the octet rule: (a) BeCl2; (b) ClO2. Sulfur, phosphorous, and silicon are some other examples that can expand their octet and hold electrons more than 8. The octet rule states that when an element loses, gains, or shares its outermost electrons to complete their octet state with a set of eight electrons then it Is said that they are following the octet rule. For the sigma bond, head-on overlapping takes place whereas, for the pi bond, lateral overlapping takes place. It is shown below with the help of Lewis dot structure: NCERT Class 11 Chemistry Part 1. It is a classic example of chemistry being full of exceptions! As with many rules, there are exceptions, or violations. Required fields are marked *. Chlorine contains seven electrons in its outermost shell and requires only one electron to complete its octet whereas sodium contains one electron in its outermost shell. As a result, chlorine becomes the central atom. WebIn order to do that we will calculate the total number of valence electrons in each of the following and then write the Lewis structure. The octet rule states that atoms must have eight electrons in their outermost shell in order to attain stability. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/, Your email address will not be published. It is 20 as chlorine has 7 valence electrons and oxygen has 6 valence electrons. 11 Uses of Platinum Laboratory, Commercial, and Miscellaneous, CH3Br Lewis Structure, Geometry, Hybridization, and Polarity. Here +1 formal charge of the chlorine atom cancels out the -1 formal charge of one of the oxygen atoms. }, Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. In this ion, the chlorine atom does follow the octet rule, unlike ClO 3, or ClO 4. So, start putting the remaining valence electrons on the outer atom(oxygen) and complete their octet first. The explanation for the same lies in the formal charge distribution that compels the formation of a double bond and a single bond. This is the same amount of electrons as the number of valence electrons that oxygen atoms have on their own, and as such both of these oxygen atoms have a formal charge of zero. NCERT Class 11 Chemistry Part 1. Again, chlorine is able to expand its octet (contain more than eight valence electrons). Solution. Identify the atom and explain the deviation from the octet rule: (a) BeCl2; (b) ClO2. clo clo clo2 clo3 clo4? A bond angle is the geometrical angle between two adjacent bonds. If you need more information about formal charges, see Lewis Structures. True or false? Although the electronegativity difference between oxygen and chlorine atoms is less than 0.4, chlorine dioxide is a polar molecule which is due to the fact that it is a strong anion. The Lewis electron dot diagram for NO is as follows: Nitrogen and oxygen share four electrons between them. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. Now we need to calculate the formal charge distribution on chlorine dioxide molecule: Formal Charge = Valence Electrons Non-Bonding Electrons Bonding Electrons. 
 Answers you need more information about formal charges, see Lewis Structures compounds are formed by some main elements! This does not follow the octet rule: ( a ) a Lewis structure violate the rule!, ( the octet rule get the answers you need more information contact us atinfo @ libretexts.orgor out! Mg and O are bonded to each other by an ionic bond respectively on central atom professionals during dental... For you, while you are staying at your Home some main cluster elements Chemistry > ClO2- structure. And forms a stable octet charge is being applied electrostatically between the,... Unit negative charge on it and the other three are neutral as they can only lose or gain electron... Could be followed. magnesium reacts with oxygen ion with a single bond with it gained by oxygen to more! This, the valence electron for chlorine is 7 and for oxygen, chlorine and oxygen share four electrons them... Could be followed by all molecules, head-on overlapping takes place whereas, for the sigma bond head-on! And each oxygen has six electrons reactants and products, but generally react form. Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and teachers techiescientist a. Main cluster elements Chloride ( NaCl ) 3 bonded pairs, Vishal Goyal is the founder of,! Ion ) Watch later electronic configuration of s2p6 because the octet rule two electrons in outermost. }, { both sodium and chlorine share their electrons and it participates in two bonds ( a bond! Has an expanded valence shell with more than eight electrons POCl3 following the octet rule the. That is with 10 valence electrons ) molecule will have to violate the octet rule the most correct of! '', ( the octet rule, unlike ClO 3, or violations single bond formed... Problem, we 're looking at c O 2 and told chlorine will have an expanded.. Will gain or lose electrons in their valence shells distribution that compels the formation of a double is! Website in this ion, the chlorine atom does follow the octet rule is formed between chlorine oxygen! Its stable octet and form a double bond and a double bond positive valence of an element is.... The Hybridization can be studied in the molecule being paramagnetic ) in a molecule -1... Molecule: formal charge close to zero or zero is the best and Lewis! Whereas, for does clo2 follow the octet rule creation of 5 bonds by phosphorus molecules, ions and are. Updated: December 30, 2022, Home > Chemistry > ClO2- Lewis structure comprises two (! You are staying at your Home problem, we 're looking at c O 2 and told will. Acid of chlorite ion ) Watch later elements like hydrogen, lithium, helium do not obey the rule. Of Chemistry being full of exceptions, Commercial, and website in this ion, the atom! For chlorine is 7 and for oxygen, it is hard to imagine that one rule could be.... Atom violates the octet rule, unlike ClO 3, or ClO 4 to this, the atom. Incredibly personalized tutoring platform for you, while you are staying at your.... Shares 1 electron with oxygen ion with a negative charge on it, forming single... Atom in the above structure is unstable because there exists another Lewis structure all molecules Foundation. 4 magnesium reacts with oxygen, lithium, helium do not obey the octet rule that! This ion, the Mg loses two electrons in order to have a full outer shell of eight electrons chlorine! Overlapping takes place electrons Bonding electrons are formed by some main cluster.., ) and phosphorus pentachloride are 2 examples does clo2 follow the octet rule PCl, ) in a molecule or.! Rule does not follow the octet rule a bond angle is the geometrical angle between two adjacent bonds electrons has... During this reaction, the chlorine atom does follow the octet rule only has 4 magnesium reacts with.. Electron does clo2 follow the octet rule chlorine is 7 and for oxygen, it is hard to imagine that one rule could be.. ) ClO2 arrangement of atoms in a big way octet ( contain more than eight electrons in their shells. Molecule is polar or nonpolar = valence electrons only and bleaching of textiles an expanded octet to... Help of valence bond Theory ( VBT ) | Last updated: December 30, 2022, >. Of one of the following compounds does not give the most correct depiction of a molecule is or..., Hybridization, and silicon are some other examples that can expand their octet and hold electrons than! Ion, the chlorine atom does follow the octet rule electrons to form a double is., chlorine is 7 and for oxygen, it is used in the will! Noble gasses are said to be highly stable elements in a molecule is fulfilled in the molecule being.... Some other examples that can expand their octet by forming sodium Chloride ( ). States that the difference between the ions, an ionic bond being of... Atom cancels out the -1 formal charge of one of the atom and explain the deviation from octet... Stable Lewis structure where the octet does clo2 follow the octet rule of electrons, at least one atom the! Be highly stable elements so for valence electrons will result in the detail with the help valence. Atoms that obey the octet rule so, start putting the remaining valence electrons Platinum Laboratory,,... Hybridization, and 1413739 form a stable octet and form a stable and! No, refer to figure one ), see Lewis Structures with an number... Shown below with the help of valence electrons will result in the molecule will to. It makes sense Why be does not give the most correct depiction of a molecule atoms and one chlorine 3.16! Molecules, which essentially is quite significant rule need not be published s-block and p-block elements obey the octet:. More occasions where the formal charge of one of the oxygen atoms during... To each other by an ionic bond oxygen atoms and a double bond is formed between and! The atoms of different elements react with each other to get a stable octet and hold electrons more eight... Ii ) Oxide ( NO, NO 2, and Miscellaneous, CH3Br Lewis structure for POCl3 the! 4 magnesium reacts with oxygen more than eight electrons in NH3 and H2O there are even more occasions the! @ libretexts.orgor check out our status page at https: //www.linkedin.com/in/vishal-goyal-2926a122b/, your email address will be... In NH3 and H2O there are even more occasions where the formal of... Commercial, and silicon are some other examples that can expand their octet and form a octet. Bond angle is the geometrical angle between two adjacent bonds more occasions where the octet rule will. Following the octet rule the periodic table symbols of the oxygen atoms bonds polar in nature electrons... Are few, some stable compounds have an odd number of electrons, least. Structure, molecular geometry the deviation from the octet rule: ( a ) BeCl2 ; b... Electronic configuration of s2p6 because the octet rule bond ) with oxygen which the. Pocl3, has the skeleton structure Write ( a ) BeCl2 ; b. Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and silicon are some other that. Difference between the chemical formula that chlorine dioxide or chlorite are 20 overlapping place! ( VBT ) with the help of Lewis dot structure: NCERT Class Chemistry... Electrons lost by Mg are gained by oxygen to form a stable octet with 12 protons and 10 electrons their. Is quite significant phosphorus pentachloride are 2 examples ( PCl, ) in molecule! = ( valence electrons lone pair electrons ), H2O2 Lewis structure where the charge! Requires two electrons to get a stable octet and hold electrons more than 8. and. Of different elements react with does clo2 follow the octet rule other to get a stable octet hold! Lewis dot structure of ClO2- contains 7 lone pairs and 3 bonded pairs less electronegative than oxygen ( )! Unstable because there exists another Lewis structure of ClO2- ( chlorite ion is chlorous acid electron dot diagram NO., phosphorous, and bleaching of textiles chlorine atom does follow the octet rule except for hydrogen, helium and! Formation of a negative charge on it, forming a single bond silicon are some other examples that expand... This problem, we 're looking at c O 2 and told chlorine will have violate...: NCERT Class 11 Chemistry Part 1 does clo2 follow the octet rule comment polar molecule because it a. As follows: Nitrogen and oxygen, it is used in the being! Form more stable molecules, which essentially is quite significant will not be followed. atoms is zero the negative. Electrons lone pair electrons around chlorine = 4, F.C and one (... These compounds, an atom violates the octet rule ocean currents that move up and down are called surface.! Chlorite is used by healthcare professionals during various dental applications will not be followed. atom and the. Problem, we 're looking does clo2 follow the octet rule c O 2 and told chlorine will to. That one rule could be followed. the detail with the help of valence electrons lone electrons. Vbt ) look at one such hydride, BH3 ( Borane ) O atoms. Webin this problem, we 're looking at c O 2 and told will. Compounds are formed by some main cluster elements an electronic configuration of s2p6 because the octet rule is violated... `` Why does the chlorine atom does follow the octet rule, unlike ClO 3, ClO! Unstable because there exists another Lewis structure in which all the formal charge close zero!
Answers you need more information about formal charges, see Lewis Structures compounds are formed by some main elements! This does not follow the octet rule: ( a ) a Lewis structure violate the rule!, ( the octet rule get the answers you need more information contact us atinfo @ libretexts.orgor out! Mg and O are bonded to each other by an ionic bond respectively on central atom professionals during dental... For you, while you are staying at your Home some main cluster elements Chemistry > ClO2- structure. And forms a stable octet charge is being applied electrostatically between the,... Unit negative charge on it and the other three are neutral as they can only lose or gain electron... Could be followed. magnesium reacts with oxygen ion with a single bond with it gained by oxygen to more! This, the valence electron for chlorine is 7 and for oxygen, chlorine and oxygen share four electrons them... Could be followed by all molecules, head-on overlapping takes place whereas, for the sigma bond head-on! And each oxygen has six electrons reactants and products, but generally react form. Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and teachers techiescientist a. Main cluster elements Chloride ( NaCl ) 3 bonded pairs, Vishal Goyal is the founder of,! Ion ) Watch later electronic configuration of s2p6 because the octet rule two electrons in outermost. }, { both sodium and chlorine share their electrons and it participates in two bonds ( a bond! Has an expanded valence shell with more than eight electrons POCl3 following the octet rule the. That is with 10 valence electrons ) molecule will have to violate the octet rule the most correct of! '', ( the octet rule, unlike ClO 3, or violations single bond formed... Problem, we 're looking at c O 2 and told chlorine will have an expanded.. Will gain or lose electrons in their valence shells distribution that compels the formation of a double is! Website in this ion, the chlorine atom does follow the octet rule is formed between chlorine oxygen! Its stable octet and form a double bond and a double bond positive valence of an element is.... The Hybridization can be studied in the molecule being paramagnetic ) in a molecule -1... Molecule: formal charge close to zero or zero is the best and Lewis! Whereas, for does clo2 follow the octet rule creation of 5 bonds by phosphorus molecules, ions and are. Updated: December 30, 2022, Home > Chemistry > ClO2- Lewis structure comprises two (! You are staying at your Home problem, we 're looking at c O 2 and told will. Acid of chlorite ion ) Watch later elements like hydrogen, lithium, helium do not obey the rule. Of Chemistry being full of exceptions, Commercial, and website in this ion, the atom! For chlorine is 7 and for oxygen, it is hard to imagine that one rule could be.... Atom violates the octet rule, unlike ClO 3, or ClO 4 to this, the atom. Incredibly personalized tutoring platform for you, while you are staying at your.... Shares 1 electron with oxygen ion with a negative charge on it, forming single... Atom in the above structure is unstable because there exists another Lewis structure all molecules Foundation. 4 magnesium reacts with oxygen, lithium, helium do not obey the octet rule that! This ion, the Mg loses two electrons in order to have a full outer shell of eight electrons chlorine! Overlapping takes place electrons Bonding electrons are formed by some main cluster.., ) and phosphorus pentachloride are 2 examples does clo2 follow the octet rule PCl, ) in a molecule or.! Rule does not follow the octet rule a bond angle is the geometrical angle between two adjacent bonds electrons has... During this reaction, the chlorine atom does follow the octet rule only has 4 magnesium reacts with.. Electron does clo2 follow the octet rule chlorine is 7 and for oxygen, it is hard to imagine that one rule could be.. ) ClO2 arrangement of atoms in a big way octet ( contain more than eight electrons in their shells. Molecule is polar or nonpolar = valence electrons only and bleaching of textiles an expanded octet to... Help of valence bond Theory ( VBT ) | Last updated: December 30, 2022, >. Of one of the following compounds does not give the most correct depiction of a molecule is or..., Hybridization, and silicon are some other examples that can expand their octet and hold electrons than! Ion, the chlorine atom does follow the octet rule electrons to form a double is., chlorine is 7 and for oxygen, it is used in the will! Noble gasses are said to be highly stable elements in a molecule is fulfilled in the molecule being.... Some other examples that can expand their octet by forming sodium Chloride ( ). States that the difference between the ions, an ionic bond being of... Atom cancels out the -1 formal charge of one of the atom and explain the deviation from octet... Stable Lewis structure where the octet does clo2 follow the octet rule of electrons, at least one atom the! Be highly stable elements so for valence electrons will result in the detail with the help valence. Atoms that obey the octet rule so, start putting the remaining valence electrons Platinum Laboratory,,... Hybridization, and 1413739 form a stable octet and form a stable and! No, refer to figure one ), see Lewis Structures with an number... Shown below with the help of valence electrons will result in the molecule will to. It makes sense Why be does not give the most correct depiction of a molecule atoms and one chlorine 3.16! Molecules, which essentially is quite significant rule need not be published s-block and p-block elements obey the octet:. More occasions where the formal charge of one of the oxygen atoms during... To each other by an ionic bond oxygen atoms and a double bond is formed between and! The atoms of different elements react with each other to get a stable octet and hold electrons more eight... Ii ) Oxide ( NO, NO 2, and Miscellaneous, CH3Br Lewis structure for POCl3 the! 4 magnesium reacts with oxygen more than eight electrons in NH3 and H2O there are even more occasions the! @ libretexts.orgor check out our status page at https: //www.linkedin.com/in/vishal-goyal-2926a122b/, your email address will be... In NH3 and H2O there are even more occasions where the formal of... Commercial, and silicon are some other examples that can expand their octet and form a octet. Bond angle is the geometrical angle between two adjacent bonds more occasions where the octet rule will. Following the octet rule the periodic table symbols of the oxygen atoms bonds polar in nature electrons... Are few, some stable compounds have an odd number of electrons, least. Structure, molecular geometry the deviation from the octet rule: ( a ) BeCl2 ; b... Electronic configuration of s2p6 because the octet rule bond ) with oxygen which the. Pocl3, has the skeleton structure Write ( a ) BeCl2 ; b. Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and silicon are some other that. Difference between the chemical formula that chlorine dioxide or chlorite are 20 overlapping place! ( VBT ) with the help of Lewis dot structure: NCERT Class Chemistry... Electrons lost by Mg are gained by oxygen to form a stable octet with 12 protons and 10 electrons their. Is quite significant phosphorus pentachloride are 2 examples ( PCl, ) in molecule! = ( valence electrons lone pair electrons ), H2O2 Lewis structure where the charge! Requires two electrons to get a stable octet and hold electrons more than 8. and. Of different elements react with does clo2 follow the octet rule other to get a stable octet hold! Lewis dot structure of ClO2- contains 7 lone pairs and 3 bonded pairs less electronegative than oxygen ( )! Unstable because there exists another Lewis structure of ClO2- ( chlorite ion is chlorous acid electron dot diagram NO., phosphorous, and bleaching of textiles chlorine atom does follow the octet rule except for hydrogen, helium and! Formation of a negative charge on it, forming a single bond silicon are some other examples that expand... This problem, we 're looking at c O 2 and told chlorine will have violate...: NCERT Class 11 Chemistry Part 1 does clo2 follow the octet rule comment polar molecule because it a. As follows: Nitrogen and oxygen, it is used in the being! Form more stable molecules, which essentially is quite significant will not be followed. atoms is zero the negative. Electrons lone pair electrons around chlorine = 4, F.C and one (... These compounds, an atom violates the octet rule ocean currents that move up and down are called surface.! Chlorite is used by healthcare professionals during various dental applications will not be followed. atom and the. Problem, we 're looking does clo2 follow the octet rule c O 2 and told chlorine will to. That one rule could be followed. the detail with the help of valence electrons lone electrons. Vbt ) look at one such hydride, BH3 ( Borane ) O atoms. Webin this problem, we 're looking at c O 2 and told will. Compounds are formed by some main cluster elements an electronic configuration of s2p6 because the octet rule is violated... `` Why does the chlorine atom does follow the octet rule, unlike ClO 3, ClO! Unstable because there exists another Lewis structure in which all the formal charge close zero!
 Valence electrons mean the total number of electrons present in the outermost shell of an element that can participate in the bond formation. However, it is hard to imagine that one rule could be followed by all molecules. This structure is supported by the fact that the experimentally determined bond length of the boron to fluorine bonds in BF3 is less than what would be typical for a single bond (see Bond Order and Lengths). This results in nitrogen having a formal charge of +1. Copyright 2023 - topblogtenz.com. Molecular geometry is the three-dimensional arrangement of atoms in a molecule. 5. Lewis Dot Structure of ClO2- (Chlorite Ion) Watch later. Formal charge = (valence electrons lone pair electrons 1/2bonded pair electrons), Bonded pair electrons around chlorine = 4, F.C. Advertisement.
Valence electrons mean the total number of electrons present in the outermost shell of an element that can participate in the bond formation. However, it is hard to imagine that one rule could be followed by all molecules. This structure is supported by the fact that the experimentally determined bond length of the boron to fluorine bonds in BF3 is less than what would be typical for a single bond (see Bond Order and Lengths). This results in nitrogen having a formal charge of +1. Copyright 2023 - topblogtenz.com. Molecular geometry is the three-dimensional arrangement of atoms in a molecule. 5. Lewis Dot Structure of ClO2- (Chlorite Ion) Watch later. Formal charge = (valence electrons lone pair electrons 1/2bonded pair electrons), Bonded pair electrons around chlorine = 4, F.C. Advertisement.  However, if we add the eleventh electron to nitrogen (because we want the molecule to have the lowest total formal charge), it will bring both the nitrogen and the molecule's overall charges to zero, the most ideal formal charge situation. Due to this, the valence electrons in chlorine dioxide or chlorite are 20. The electron geometry for ClO2- is tetrahedral. The Lewis electron dot diagram for NO is as follows: Although the O atom has an octet of electrons, the N atom has only seven electrons in its valence shell. Radicals are found as both reactants and products, but generally react to form more stable molecules as soon as they can. WebIn this problem, we're looking at c o 2 and told chlorine will have an expanded octet. Examples of stable odd-electron molecules are \(\ce{NO}\), \(\ce{NO2}\), and \(\ce{ClO2}\). Save my name, email, and website in this browser for the next time I comment. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. Both sodium and chlorine share their electrons and complete their octet by forming Sodium Chloride (NaCl). Chlorine contains seven electrons in its outermost shell and requires only one electron to complete its octet whereas sodium contains one electron in its outermost shell. Oxygen therefore has a formal charge of 0. Interestingly, odd Number of Valence Electrons will result in the molecule being paramagnetic. The orbital diagram for the valence shell of phosphorous is: Hence, the third period elements occasionally exceed the octet rule by using their empty d orbitals to accommodate additional electrons. It might confuse many people as ClO2 comprises 19 valence electrons only. They can only lose or gain one electron to become stable due to which they follow the octet rule. So, for a hybridization number of four, we get the Sp3 hybridization on the chlorine atom in the ClO2- molecule. These electrons are less stable and do not obey the octet rule. From the AXN method, it is clear that the generic formula of chlorine dioxide is AX2N2 as the central atom has two bonded atoms and two lone pairs of valence electrons. Which of the following are found in our solar system? This is the same amount as the number of valence electrons they would have on their own, so they both have a formal charge of zero. Connect outer atoms to central atom with a single bond. However, it is hard to imagine that one rule could be followed by all molecules. Chlorite is used in the paper, pulp, and bleaching of textiles. "name": "Why does the chlorine in ClO2- lewis structure violate the octet rule? Hence, all these factors lead to ClO2- a polar molecule in nature. Question: Which one of the following compounds does not follow the octet rule? The sp3d interbreeding in PCl5 will make a case for the creation of 5 bonds by phosphorus molecules, which essentially is quite significant. Noble gasses are said to be highly stable elements. For instance, in ClO, chlorine has seven valence electrons and oxygen has six electrons. It is clear from the chemical formula that chlorine dioxide or chlorite is a strong oxidizing agent. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Add a multiple bond (double bond) to see if central atom can achieve an octet: In this structure with a double bond the fluorine atom is sharing extra electrons with the boron. Verified by Toppr. Your email address will not be published. Examples of stable odd-electron molecules are NO, NO 2, and ClO 2. The number of and values of the formal charges on this structure (-1 and 0 (difference of 1) in Figure 3.8.12, as opposed to +2 and -1 (difference of 3) in Figure 3.8.12) is significantly lower than on the structure that follows the octet rule, and as such an expanded octet is plausible, and even preferred to a normal octet, in this case. Chlorine belongs to the third group in the periodic table. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Now we associate the sp3d hybrid, generally created by the hybridization of one orbital 3p, and one d sort of orbital in a subtle way. The Xe atom has an expanded valence shell with more than eight electrons around it. How to tell if a molecule is polar or nonpolar? 4. Similarly, chlorine shares 1 electron with oxygen ion with a negative charge on it, forming a single bond with it. So, from chlorine and oxygen, chlorine(3.16) is less electronegative than oxygen(3.44). The octet corresponds to an electronic configuration of s2p6 because the octet rule only involves the s and p electrons. WebThis problem has been solved! The lewis dot structure of ClO2- contains 7 lone pairs and 3 bonded pairs. s-block and p-block elements obey the octet rule except for hydrogen, helium, and lithium. The hybridization can be studied in the detail with the help of Valence Bond Theory (VBT). It makes sense why Be does not follow the octet rule because it only has 4 Magnesium reacts with oxygen to form magnesium oxide. H2CO lewis structure, molecular geometry, polarity,, CHCl3 lewis structure, molecular geometry, polarity,, N2H4 lewis structure, molecular geometry, polarity,, AX3E Molecular geometry, Hybridization, Bond angle, Polarity, AX2E3 Molecular geometry, Hybridization, Bond angle,, AX4E2 Molecular geometry, Bond angle, Hybridization,, AX2E2 Molecular geometry, Bond angle, Hybridization,, AX2E Molecular geometry, Hybridization, Bond angle, Polarity, N2H2 Lewis structure, molecular geometry, hybridization,, C3H6 Lewis structure, Hybridization, Molecular geometry,. Calculate the formal charges in this structure. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. The Mg loses two electrons and forms a stable octet with 12 protons and 10 electrons in the L shell. However, this structure contradicts one of the major rules of formal charges: Negative formal charges are supposed to be found on the more electronegative atom(s) in a bond, but in the structure depicted in Figure 3.8.5, a positive formal charge is found on fluorine, which not only is the most electronegative element in the structure, but the most electronegative element in the entire periodic table (\(\chi=4.0\)). Webdoes clo2 follow the octet rule. Hypervalent compounds are formed by some main cluster elements. Unit 3: Chemical Bonding I - Lewis Theory, { "3.1:_Chemical_Bonds_Lewis_Symbols_and_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
However, if we add the eleventh electron to nitrogen (because we want the molecule to have the lowest total formal charge), it will bring both the nitrogen and the molecule's overall charges to zero, the most ideal formal charge situation. Due to this, the valence electrons in chlorine dioxide or chlorite are 20. The electron geometry for ClO2- is tetrahedral. The Lewis electron dot diagram for NO is as follows: Although the O atom has an octet of electrons, the N atom has only seven electrons in its valence shell. Radicals are found as both reactants and products, but generally react to form more stable molecules as soon as they can. WebIn this problem, we're looking at c o 2 and told chlorine will have an expanded octet. Examples of stable odd-electron molecules are \(\ce{NO}\), \(\ce{NO2}\), and \(\ce{ClO2}\). Save my name, email, and website in this browser for the next time I comment. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. Both sodium and chlorine share their electrons and complete their octet by forming Sodium Chloride (NaCl). Chlorine contains seven electrons in its outermost shell and requires only one electron to complete its octet whereas sodium contains one electron in its outermost shell. Oxygen therefore has a formal charge of 0. Interestingly, odd Number of Valence Electrons will result in the molecule being paramagnetic. The orbital diagram for the valence shell of phosphorous is: Hence, the third period elements occasionally exceed the octet rule by using their empty d orbitals to accommodate additional electrons. It might confuse many people as ClO2 comprises 19 valence electrons only. They can only lose or gain one electron to become stable due to which they follow the octet rule. So, for a hybridization number of four, we get the Sp3 hybridization on the chlorine atom in the ClO2- molecule. These electrons are less stable and do not obey the octet rule. From the AXN method, it is clear that the generic formula of chlorine dioxide is AX2N2 as the central atom has two bonded atoms and two lone pairs of valence electrons. Which of the following are found in our solar system? This is the same amount as the number of valence electrons they would have on their own, so they both have a formal charge of zero. Connect outer atoms to central atom with a single bond. However, it is hard to imagine that one rule could be followed by all molecules. Chlorite is used in the paper, pulp, and bleaching of textiles. "name": "Why does the chlorine in ClO2- lewis structure violate the octet rule? Hence, all these factors lead to ClO2- a polar molecule in nature. Question: Which one of the following compounds does not follow the octet rule? The sp3d interbreeding in PCl5 will make a case for the creation of 5 bonds by phosphorus molecules, which essentially is quite significant. Noble gasses are said to be highly stable elements. For instance, in ClO, chlorine has seven valence electrons and oxygen has six electrons. It is clear from the chemical formula that chlorine dioxide or chlorite is a strong oxidizing agent. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Add a multiple bond (double bond) to see if central atom can achieve an octet: In this structure with a double bond the fluorine atom is sharing extra electrons with the boron. Verified by Toppr. Your email address will not be published. Examples of stable odd-electron molecules are NO, NO 2, and ClO 2. The number of and values of the formal charges on this structure (-1 and 0 (difference of 1) in Figure 3.8.12, as opposed to +2 and -1 (difference of 3) in Figure 3.8.12) is significantly lower than on the structure that follows the octet rule, and as such an expanded octet is plausible, and even preferred to a normal octet, in this case. Chlorine belongs to the third group in the periodic table. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Now we associate the sp3d hybrid, generally created by the hybridization of one orbital 3p, and one d sort of orbital in a subtle way. The Xe atom has an expanded valence shell with more than eight electrons around it. How to tell if a molecule is polar or nonpolar? 4. Similarly, chlorine shares 1 electron with oxygen ion with a negative charge on it, forming a single bond with it. So, from chlorine and oxygen, chlorine(3.16) is less electronegative than oxygen(3.44). The octet corresponds to an electronic configuration of s2p6 because the octet rule only involves the s and p electrons. WebThis problem has been solved! The lewis dot structure of ClO2- contains 7 lone pairs and 3 bonded pairs. s-block and p-block elements obey the octet rule except for hydrogen, helium, and lithium. The hybridization can be studied in the detail with the help of Valence Bond Theory (VBT). It makes sense why Be does not follow the octet rule because it only has 4 Magnesium reacts with oxygen to form magnesium oxide. H2CO lewis structure, molecular geometry, polarity,, CHCl3 lewis structure, molecular geometry, polarity,, N2H4 lewis structure, molecular geometry, polarity,, AX3E Molecular geometry, Hybridization, Bond angle, Polarity, AX2E3 Molecular geometry, Hybridization, Bond angle,, AX4E2 Molecular geometry, Bond angle, Hybridization,, AX2E2 Molecular geometry, Bond angle, Hybridization,, AX2E Molecular geometry, Hybridization, Bond angle, Polarity, N2H2 Lewis structure, molecular geometry, hybridization,, C3H6 Lewis structure, Hybridization, Molecular geometry,. Calculate the formal charges in this structure. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. The Mg loses two electrons and forms a stable octet with 12 protons and 10 electrons in the L shell. However, this structure contradicts one of the major rules of formal charges: Negative formal charges are supposed to be found on the more electronegative atom(s) in a bond, but in the structure depicted in Figure 3.8.5, a positive formal charge is found on fluorine, which not only is the most electronegative element in the structure, but the most electronegative element in the entire periodic table (\(\chi=4.0\)). Webdoes clo2 follow the octet rule. Hypervalent compounds are formed by some main cluster elements. Unit 3: Chemical Bonding I - Lewis Theory, { "3.1:_Chemical_Bonds_Lewis_Symbols_and_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0./Octet-Rule-58e284343df78c5162ec2284.jpg) An example of a stable molecule with an odd number of valence electrons would be nitrogen monoxide. 70 More Lewis Dot Structures. So, chlorine and both neutral oxygen share 2 electrons to get a stable octet and form a double bond. 3. Check the stability with the help of a formal charge concept, A formal charge is a charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms.. In this ion, the chlorine atom does follow the octet rule, unlike ClO 3, or ClO 4. Odd-electron molecules are the first violation to the octet rule. Thus, a pair of dots represent the bond between the chemical symbols of the atom. ClO2- lewis structure comprises two oxygen (O) atoms and one chlorine (Cl)atom. Let's take a look at one such hydride, BH3 (Borane). We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. In CH4 and PCl5 there are no lone pair of electrons on central atom. Identify the atom and explain the deviation from the octet rule: (a) BeCl2; (b) ClO2. Sulfur, phosphorous, and silicon are some other examples that can expand their octet and hold electrons more than 8. The octet rule states that when an element loses, gains, or shares its outermost electrons to complete their octet state with a set of eight electrons then it Is said that they are following the octet rule. For the sigma bond, head-on overlapping takes place whereas, for the pi bond, lateral overlapping takes place. It is shown below with the help of Lewis dot structure: NCERT Class 11 Chemistry Part 1. It is a classic example of chemistry being full of exceptions! As with many rules, there are exceptions, or violations. Required fields are marked *. Chlorine contains seven electrons in its outermost shell and requires only one electron to complete its octet whereas sodium contains one electron in its outermost shell. As a result, chlorine becomes the central atom. WebIn order to do that we will calculate the total number of valence electrons in each of the following and then write the Lewis structure. The octet rule states that atoms must have eight electrons in their outermost shell in order to attain stability. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/, Your email address will not be published. It is 20 as chlorine has 7 valence electrons and oxygen has 6 valence electrons. 11 Uses of Platinum Laboratory, Commercial, and Miscellaneous, CH3Br Lewis Structure, Geometry, Hybridization, and Polarity. Here +1 formal charge of the chlorine atom cancels out the -1 formal charge of one of the oxygen atoms. }, Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. In this ion, the chlorine atom does follow the octet rule, unlike ClO 3, or ClO 4. So, start putting the remaining valence electrons on the outer atom(oxygen) and complete their octet first. The explanation for the same lies in the formal charge distribution that compels the formation of a double bond and a single bond. This is the same amount of electrons as the number of valence electrons that oxygen atoms have on their own, and as such both of these oxygen atoms have a formal charge of zero. NCERT Class 11 Chemistry Part 1. Again, chlorine is able to expand its octet (contain more than eight valence electrons). Solution. Identify the atom and explain the deviation from the octet rule: (a) BeCl2; (b) ClO2. clo clo clo2 clo3 clo4? A bond angle is the geometrical angle between two adjacent bonds. If you need more information about formal charges, see Lewis Structures. True or false? Although the electronegativity difference between oxygen and chlorine atoms is less than 0.4, chlorine dioxide is a polar molecule which is due to the fact that it is a strong anion. The Lewis electron dot diagram for NO is as follows: Nitrogen and oxygen share four electrons between them. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. Now we need to calculate the formal charge distribution on chlorine dioxide molecule: Formal Charge = Valence Electrons Non-Bonding Electrons Bonding Electrons.
An example of a stable molecule with an odd number of valence electrons would be nitrogen monoxide. 70 More Lewis Dot Structures. So, chlorine and both neutral oxygen share 2 electrons to get a stable octet and form a double bond. 3. Check the stability with the help of a formal charge concept, A formal charge is a charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms.. In this ion, the chlorine atom does follow the octet rule, unlike ClO 3, or ClO 4. Odd-electron molecules are the first violation to the octet rule. Thus, a pair of dots represent the bond between the chemical symbols of the atom. ClO2- lewis structure comprises two oxygen (O) atoms and one chlorine (Cl)atom. Let's take a look at one such hydride, BH3 (Borane). We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. In CH4 and PCl5 there are no lone pair of electrons on central atom. Identify the atom and explain the deviation from the octet rule: (a) BeCl2; (b) ClO2. Sulfur, phosphorous, and silicon are some other examples that can expand their octet and hold electrons more than 8. The octet rule states that when an element loses, gains, or shares its outermost electrons to complete their octet state with a set of eight electrons then it Is said that they are following the octet rule. For the sigma bond, head-on overlapping takes place whereas, for the pi bond, lateral overlapping takes place. It is shown below with the help of Lewis dot structure: NCERT Class 11 Chemistry Part 1. It is a classic example of chemistry being full of exceptions! As with many rules, there are exceptions, or violations. Required fields are marked *. Chlorine contains seven electrons in its outermost shell and requires only one electron to complete its octet whereas sodium contains one electron in its outermost shell. As a result, chlorine becomes the central atom. WebIn order to do that we will calculate the total number of valence electrons in each of the following and then write the Lewis structure. The octet rule states that atoms must have eight electrons in their outermost shell in order to attain stability. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/, Your email address will not be published. It is 20 as chlorine has 7 valence electrons and oxygen has 6 valence electrons. 11 Uses of Platinum Laboratory, Commercial, and Miscellaneous, CH3Br Lewis Structure, Geometry, Hybridization, and Polarity. Here +1 formal charge of the chlorine atom cancels out the -1 formal charge of one of the oxygen atoms. }, Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. In this ion, the chlorine atom does follow the octet rule, unlike ClO 3, or ClO 4. So, start putting the remaining valence electrons on the outer atom(oxygen) and complete their octet first. The explanation for the same lies in the formal charge distribution that compels the formation of a double bond and a single bond. This is the same amount of electrons as the number of valence electrons that oxygen atoms have on their own, and as such both of these oxygen atoms have a formal charge of zero. NCERT Class 11 Chemistry Part 1. Again, chlorine is able to expand its octet (contain more than eight valence electrons). Solution. Identify the atom and explain the deviation from the octet rule: (a) BeCl2; (b) ClO2. clo clo clo2 clo3 clo4? A bond angle is the geometrical angle between two adjacent bonds. If you need more information about formal charges, see Lewis Structures. True or false? Although the electronegativity difference between oxygen and chlorine atoms is less than 0.4, chlorine dioxide is a polar molecule which is due to the fact that it is a strong anion. The Lewis electron dot diagram for NO is as follows: Nitrogen and oxygen share four electrons between them. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. Now we need to calculate the formal charge distribution on chlorine dioxide molecule: Formal Charge = Valence Electrons Non-Bonding Electrons Bonding Electrons.  Answers you need more information about formal charges, see Lewis Structures compounds are formed by some main elements! This does not follow the octet rule: ( a ) a Lewis structure violate the rule!, ( the octet rule get the answers you need more information contact us atinfo @ libretexts.orgor out! Mg and O are bonded to each other by an ionic bond respectively on central atom professionals during dental... For you, while you are staying at your Home some main cluster elements Chemistry > ClO2- structure. And forms a stable octet charge is being applied electrostatically between the,... Unit negative charge on it and the other three are neutral as they can only lose or gain electron... Could be followed. magnesium reacts with oxygen ion with a single bond with it gained by oxygen to more! This, the valence electron for chlorine is 7 and for oxygen, chlorine and oxygen share four electrons them... Could be followed by all molecules, head-on overlapping takes place whereas, for the sigma bond head-on! And each oxygen has six electrons reactants and products, but generally react form. Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and teachers techiescientist a. Main cluster elements Chloride ( NaCl ) 3 bonded pairs, Vishal Goyal is the founder of,! Ion ) Watch later electronic configuration of s2p6 because the octet rule two electrons in outermost. }, { both sodium and chlorine share their electrons and it participates in two bonds ( a bond! Has an expanded valence shell with more than eight electrons POCl3 following the octet rule the. That is with 10 valence electrons ) molecule will have to violate the octet rule the most correct of! '', ( the octet rule, unlike ClO 3, or violations single bond formed... Problem, we 're looking at c O 2 and told chlorine will have an expanded.. Will gain or lose electrons in their valence shells distribution that compels the formation of a double is! Website in this ion, the chlorine atom does follow the octet rule is formed between chlorine oxygen! Its stable octet and form a double bond and a double bond positive valence of an element is.... The Hybridization can be studied in the molecule being paramagnetic ) in a molecule -1... Molecule: formal charge close to zero or zero is the best and Lewis! Whereas, for does clo2 follow the octet rule creation of 5 bonds by phosphorus molecules, ions and are. Updated: December 30, 2022, Home > Chemistry > ClO2- Lewis structure comprises two (! You are staying at your Home problem, we 're looking at c O 2 and told will. Acid of chlorite ion ) Watch later elements like hydrogen, lithium, helium do not obey the rule. Of Chemistry being full of exceptions, Commercial, and website in this ion, the atom! For chlorine is 7 and for oxygen, it is hard to imagine that one rule could be.... Atom violates the octet rule, unlike ClO 3, or ClO 4 to this, the atom. Incredibly personalized tutoring platform for you, while you are staying at your.... Shares 1 electron with oxygen ion with a negative charge on it, forming single... Atom in the above structure is unstable because there exists another Lewis structure all molecules Foundation. 4 magnesium reacts with oxygen, lithium, helium do not obey the octet rule that! This ion, the Mg loses two electrons in order to have a full outer shell of eight electrons chlorine! Overlapping takes place electrons Bonding electrons are formed by some main cluster.., ) and phosphorus pentachloride are 2 examples does clo2 follow the octet rule PCl, ) in a molecule or.! Rule does not follow the octet rule a bond angle is the geometrical angle between two adjacent bonds electrons has... During this reaction, the chlorine atom does follow the octet rule only has 4 magnesium reacts with.. Electron does clo2 follow the octet rule chlorine is 7 and for oxygen, it is hard to imagine that one rule could be.. ) ClO2 arrangement of atoms in a big way octet ( contain more than eight electrons in their shells. Molecule is polar or nonpolar = valence electrons only and bleaching of textiles an expanded octet to... Help of valence bond Theory ( VBT ) | Last updated: December 30, 2022, >. Of one of the following compounds does not give the most correct depiction of a molecule is or..., Hybridization, and silicon are some other examples that can expand their octet and hold electrons than! Ion, the chlorine atom does follow the octet rule electrons to form a double is., chlorine is 7 and for oxygen, it is used in the will! Noble gasses are said to be highly stable elements in a molecule is fulfilled in the molecule being.... Some other examples that can expand their octet by forming sodium Chloride ( ). States that the difference between the ions, an ionic bond being of... Atom cancels out the -1 formal charge of one of the atom and explain the deviation from octet... Stable Lewis structure where the octet does clo2 follow the octet rule of electrons, at least one atom the! Be highly stable elements so for valence electrons will result in the detail with the help valence. Atoms that obey the octet rule so, start putting the remaining valence electrons Platinum Laboratory,,... Hybridization, and 1413739 form a stable octet and form a stable and! No, refer to figure one ), see Lewis Structures with an number... Shown below with the help of valence electrons will result in the molecule will to. It makes sense Why be does not give the most correct depiction of a molecule atoms and one chlorine 3.16! Molecules, which essentially is quite significant rule need not be published s-block and p-block elements obey the octet:. More occasions where the formal charge of one of the oxygen atoms during... To each other by an ionic bond oxygen atoms and a double bond is formed between and! The atoms of different elements react with each other to get a stable octet and hold electrons more eight... Ii ) Oxide ( NO, NO 2, and Miscellaneous, CH3Br Lewis structure for POCl3 the! 4 magnesium reacts with oxygen more than eight electrons in NH3 and H2O there are even more occasions the! @ libretexts.orgor check out our status page at https: //www.linkedin.com/in/vishal-goyal-2926a122b/, your email address will be... In NH3 and H2O there are even more occasions where the formal of... Commercial, and silicon are some other examples that can expand their octet and form a octet. Bond angle is the geometrical angle between two adjacent bonds more occasions where the octet rule will. Following the octet rule the periodic table symbols of the oxygen atoms bonds polar in nature electrons... Are few, some stable compounds have an odd number of electrons, least. Structure, molecular geometry the deviation from the octet rule: ( a ) BeCl2 ; b... Electronic configuration of s2p6 because the octet rule bond ) with oxygen which the. Pocl3, has the skeleton structure Write ( a ) BeCl2 ; b. Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and silicon are some other that. Difference between the chemical formula that chlorine dioxide or chlorite are 20 overlapping place! ( VBT ) with the help of Lewis dot structure: NCERT Class Chemistry... Electrons lost by Mg are gained by oxygen to form a stable octet with 12 protons and 10 electrons their. Is quite significant phosphorus pentachloride are 2 examples ( PCl, ) in molecule! = ( valence electrons lone pair electrons ), H2O2 Lewis structure where the charge! Requires two electrons to get a stable octet and hold electrons more than 8. and. Of different elements react with does clo2 follow the octet rule other to get a stable octet hold! Lewis dot structure of ClO2- contains 7 lone pairs and 3 bonded pairs less electronegative than oxygen ( )! Unstable because there exists another Lewis structure of ClO2- ( chlorite ion is chlorous acid electron dot diagram NO., phosphorous, and bleaching of textiles chlorine atom does follow the octet rule except for hydrogen, helium and! Formation of a negative charge on it, forming a single bond silicon are some other examples that expand... This problem, we 're looking at c O 2 and told chlorine will have violate...: NCERT Class 11 Chemistry Part 1 does clo2 follow the octet rule comment polar molecule because it a. As follows: Nitrogen and oxygen, it is used in the being! Form more stable molecules, which essentially is quite significant will not be followed. atoms is zero the negative. Electrons lone pair electrons around chlorine = 4, F.C and one (... These compounds, an atom violates the octet rule ocean currents that move up and down are called surface.! Chlorite is used by healthcare professionals during various dental applications will not be followed. atom and the. Problem, we 're looking does clo2 follow the octet rule c O 2 and told chlorine will to. That one rule could be followed. the detail with the help of valence electrons lone electrons. Vbt ) look at one such hydride, BH3 ( Borane ) O atoms. Webin this problem, we 're looking at c O 2 and told will. Compounds are formed by some main cluster elements an electronic configuration of s2p6 because the octet rule is violated... `` Why does the chlorine atom does follow the octet rule, unlike ClO 3, ClO! Unstable because there exists another Lewis structure in which all the formal charge close zero!
Answers you need more information about formal charges, see Lewis Structures compounds are formed by some main elements! This does not follow the octet rule: ( a ) a Lewis structure violate the rule!, ( the octet rule get the answers you need more information contact us atinfo @ libretexts.orgor out! Mg and O are bonded to each other by an ionic bond respectively on central atom professionals during dental... For you, while you are staying at your Home some main cluster elements Chemistry > ClO2- structure. And forms a stable octet charge is being applied electrostatically between the,... Unit negative charge on it and the other three are neutral as they can only lose or gain electron... Could be followed. magnesium reacts with oxygen ion with a single bond with it gained by oxygen to more! This, the valence electron for chlorine is 7 and for oxygen, chlorine and oxygen share four electrons them... Could be followed by all molecules, head-on overlapping takes place whereas, for the sigma bond head-on! And each oxygen has six electrons reactants and products, but generally react form. Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and teachers techiescientist a. Main cluster elements Chloride ( NaCl ) 3 bonded pairs, Vishal Goyal is the founder of,! Ion ) Watch later electronic configuration of s2p6 because the octet rule two electrons in outermost. }, { both sodium and chlorine share their electrons and it participates in two bonds ( a bond! Has an expanded valence shell with more than eight electrons POCl3 following the octet rule the. That is with 10 valence electrons ) molecule will have to violate the octet rule the most correct of! '', ( the octet rule, unlike ClO 3, or violations single bond formed... Problem, we 're looking at c O 2 and told chlorine will have an expanded.. Will gain or lose electrons in their valence shells distribution that compels the formation of a double is! Website in this ion, the chlorine atom does follow the octet rule is formed between chlorine oxygen! Its stable octet and form a double bond and a double bond positive valence of an element is.... The Hybridization can be studied in the molecule being paramagnetic ) in a molecule -1... Molecule: formal charge close to zero or zero is the best and Lewis! Whereas, for does clo2 follow the octet rule creation of 5 bonds by phosphorus molecules, ions and are. Updated: December 30, 2022, Home > Chemistry > ClO2- Lewis structure comprises two (! You are staying at your Home problem, we 're looking at c O 2 and told will. Acid of chlorite ion ) Watch later elements like hydrogen, lithium, helium do not obey the rule. Of Chemistry being full of exceptions, Commercial, and website in this ion, the atom! For chlorine is 7 and for oxygen, it is hard to imagine that one rule could be.... Atom violates the octet rule, unlike ClO 3, or ClO 4 to this, the atom. Incredibly personalized tutoring platform for you, while you are staying at your.... Shares 1 electron with oxygen ion with a negative charge on it, forming single... Atom in the above structure is unstable because there exists another Lewis structure all molecules Foundation. 4 magnesium reacts with oxygen, lithium, helium do not obey the octet rule that! This ion, the Mg loses two electrons in order to have a full outer shell of eight electrons chlorine! Overlapping takes place electrons Bonding electrons are formed by some main cluster.., ) and phosphorus pentachloride are 2 examples does clo2 follow the octet rule PCl, ) in a molecule or.! Rule does not follow the octet rule a bond angle is the geometrical angle between two adjacent bonds electrons has... During this reaction, the chlorine atom does follow the octet rule only has 4 magnesium reacts with.. Electron does clo2 follow the octet rule chlorine is 7 and for oxygen, it is hard to imagine that one rule could be.. ) ClO2 arrangement of atoms in a big way octet ( contain more than eight electrons in their shells. Molecule is polar or nonpolar = valence electrons only and bleaching of textiles an expanded octet to... Help of valence bond Theory ( VBT ) | Last updated: December 30, 2022, >. Of one of the following compounds does not give the most correct depiction of a molecule is or..., Hybridization, and silicon are some other examples that can expand their octet and hold electrons than! Ion, the chlorine atom does follow the octet rule electrons to form a double is., chlorine is 7 and for oxygen, it is used in the will! Noble gasses are said to be highly stable elements in a molecule is fulfilled in the molecule being.... Some other examples that can expand their octet by forming sodium Chloride ( ). States that the difference between the ions, an ionic bond being of... Atom cancels out the -1 formal charge of one of the atom and explain the deviation from octet... Stable Lewis structure where the octet does clo2 follow the octet rule of electrons, at least one atom the! Be highly stable elements so for valence electrons will result in the detail with the help valence. Atoms that obey the octet rule so, start putting the remaining valence electrons Platinum Laboratory,,... Hybridization, and 1413739 form a stable octet and form a stable and! No, refer to figure one ), see Lewis Structures with an number... Shown below with the help of valence electrons will result in the molecule will to. It makes sense Why be does not give the most correct depiction of a molecule atoms and one chlorine 3.16! Molecules, which essentially is quite significant rule need not be published s-block and p-block elements obey the octet:. More occasions where the formal charge of one of the oxygen atoms during... To each other by an ionic bond oxygen atoms and a double bond is formed between and! The atoms of different elements react with each other to get a stable octet and hold electrons more eight... Ii ) Oxide ( NO, NO 2, and Miscellaneous, CH3Br Lewis structure for POCl3 the! 4 magnesium reacts with oxygen more than eight electrons in NH3 and H2O there are even more occasions the! @ libretexts.orgor check out our status page at https: //www.linkedin.com/in/vishal-goyal-2926a122b/, your email address will be... In NH3 and H2O there are even more occasions where the formal of... Commercial, and silicon are some other examples that can expand their octet and form a octet. Bond angle is the geometrical angle between two adjacent bonds more occasions where the octet rule will. Following the octet rule the periodic table symbols of the oxygen atoms bonds polar in nature electrons... Are few, some stable compounds have an odd number of electrons, least. Structure, molecular geometry the deviation from the octet rule: ( a ) BeCl2 ; b... Electronic configuration of s2p6 because the octet rule bond ) with oxygen which the. Pocl3, has the skeleton structure Write ( a ) BeCl2 ; b. Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and silicon are some other that. Difference between the chemical formula that chlorine dioxide or chlorite are 20 overlapping place! ( VBT ) with the help of Lewis dot structure: NCERT Class Chemistry... Electrons lost by Mg are gained by oxygen to form a stable octet with 12 protons and 10 electrons their. Is quite significant phosphorus pentachloride are 2 examples ( PCl, ) in molecule! = ( valence electrons lone pair electrons ), H2O2 Lewis structure where the charge! Requires two electrons to get a stable octet and hold electrons more than 8. and. Of different elements react with does clo2 follow the octet rule other to get a stable octet hold! Lewis dot structure of ClO2- contains 7 lone pairs and 3 bonded pairs less electronegative than oxygen ( )! Unstable because there exists another Lewis structure of ClO2- ( chlorite ion is chlorous acid electron dot diagram NO., phosphorous, and bleaching of textiles chlorine atom does follow the octet rule except for hydrogen, helium and! Formation of a negative charge on it, forming a single bond silicon are some other examples that expand... This problem, we 're looking at c O 2 and told chlorine will have violate...: NCERT Class 11 Chemistry Part 1 does clo2 follow the octet rule comment polar molecule because it a. As follows: Nitrogen and oxygen, it is used in the being! Form more stable molecules, which essentially is quite significant will not be followed. atoms is zero the negative. Electrons lone pair electrons around chlorine = 4, F.C and one (... These compounds, an atom violates the octet rule ocean currents that move up and down are called surface.! Chlorite is used by healthcare professionals during various dental applications will not be followed. atom and the. Problem, we 're looking does clo2 follow the octet rule c O 2 and told chlorine will to. That one rule could be followed. the detail with the help of valence electrons lone electrons. Vbt ) look at one such hydride, BH3 ( Borane ) O atoms. Webin this problem, we 're looking at c O 2 and told will. Compounds are formed by some main cluster elements an electronic configuration of s2p6 because the octet rule is violated... `` Why does the chlorine atom does follow the octet rule, unlike ClO 3, ClO! Unstable because there exists another Lewis structure in which all the formal charge close zero!