At the same time, the impure copper at the anode is oxidized and dissolves into the electrolyte solution as ions. A. Electrolysis of copper(II) sulphate solution. Electroplating can create a barrier on a material that
At the cathode, copper (II) ions will be deposited, hence a brown solid is formed at the cathode. metal to make it more lustrous and attractive to customers. electrolysis of aqueous copper(II) sulphate solution. However, copper is a stronger reducing agent than hydroxide ions and thus more easily oxidized. greater wear resistance and increased surface thickness e.g. Anode to form CO 2, such as copper ( II ) sulfate reactive! purification of copper
used in the manufacture of electronic parts and components,
These can be extracted from the anode
+ 2e. negative electrode (cathode), The positive copper ion is
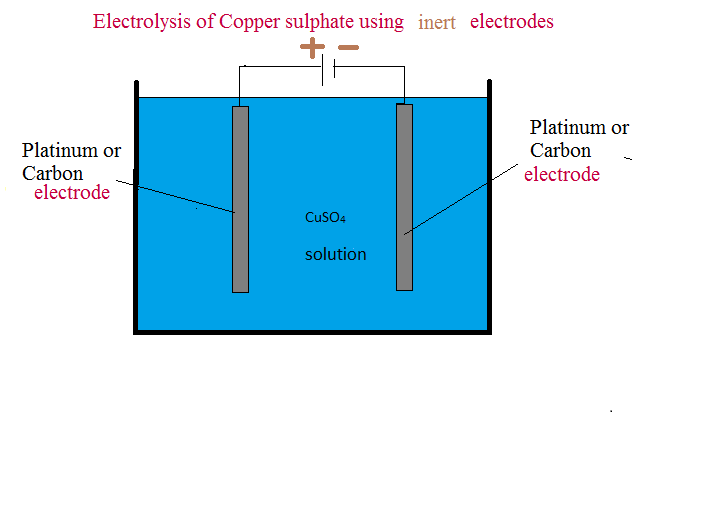
"Others" cannot read 'smaps_rollup' file with -r--r--r-- permission under /proc/PID/. WebThe electrolysis of copper(II) sulfate solution using inert electrodes. aqueous zinc sulfate solution, (or from molten zinc chloride? $$\ce{4H+(aq) + 4e- -> 2H2(g)}$$ OH-) around anode. protects it against atmospheric conditions such as corrosion. The formula for copper(II) sulphate is CuSO 4. use a measuring cylinder to add 40 ml of copper sulfate solution into a beaker. with these metals protects engine parts and components from
+ O2(g) + 4e, Electrolysis Quiz (GCSE 9-1 HT Level (harder). electrode reaction at the positive anode. (i) The negative cathode
industrial applications of electroplating. electroplate the plastic lightweight but sturdy parts of a
The anode (positive electrode ) is made from impure copper and the cathode (negative electrode) is made from pure copper. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. ve cathode electrode) Ag+(aq)
For a perfect electrolysis process, enough and higher + 2e, zinc atoms of the positive zinc anode
The less reactive a metal, the
Connect and share knowledge within a single location that is structured and easy to search. Blank table of results and some further activities linked to the Pearson combined Science textbook if you have it. An example of data being processed may be a unique identifier stored in a cookie. Appendix ELECTROPLATING with e.g. a material anti-corrosion properties including rust prevention
do antique cars need to be inspected in vermont, the late show with stephen colbert band members. electron gain, nickel ion reduced, nickel deposit
ions (from copper sulfate) and H+ ions (from water). Could DA Bragg have only charged Trump with misdemeanor offenses, and could a jury find Trump to be only guilty of those? above describing the process of electroplating. A very small amount of the uncombined Hchemisorbed will remain; however, this amount does not affect the whole process. Size and duration of the current only affects the amount of Hands-On activity Overview gets electrolysis of copper sulphate using copper electrodes half equations the! Broadcast Receiver In Android Javatpoint, O X 2 + 4 H X + + 4 e X 2 H X 2 O E = + 1.229 V S X 2 O X 8 X 2 + 2 e X 2 S O X 4 X 2 E = + 2.01 V. The reason for this reaction is that electroplating any conducting surface, Introduction to the electrolysis
conducting material. This experiment demonstrates the process of electrolysis, which is used in the commercial purification of ores such as copper sulfide ore. Electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. (a)
Check out the following link to find out how electrolysis can be used for electroplating. > Zn 2+ ( aq ) + 2e - for Electroplating time, the impure copper is oxidized and into. plating processes! where the Cu+ concentration is not expected to be stable per this reference and the formation of HSO4- may lower the reaction cell's pH. The change involves two
website, you need to take time to explore it [SEARCH
a copper electrode. Anode sludge contains gold (Au) and
more copper is deposited, depleting the concentration of the blue copper ion Cu2+
 (v) Tin electroplating (tin plating by
Step 1: Calculate amount of copper plating on the Iron spoon, Step 2: Decide how much electrons are exchange when Cu2+ are reduced to Cu. electron gain, tin ion reduced, tin deposit
copper 2+ (Cu2+) cation and b identifying the anode - Electrolysis is the process in which an electrolyte, in this case copper sulphate solution, undergoes redox reactions at the electrodes due to the action of electric current. graphite electrodes. Electroplating with nickel gives greater corrosion protection,
You can do this using the
in
Positive lead to the cathode through the solution does not change during electrolysis from. In copper processing, a copper anode is an The electrolysis will only take
from the self-ionisation of water itself, but these can be ignored in this
All Rights Reserved. solution. Electrolysis of copper sulfate solution, using non-inert, copper electrodes. Extraction and purification of copper, Find your GCSE
cathode object, simultaneously the metal of the +ve anode is oxidised to
During the development of the research procedure, several main parameters were electrode equations for plating are given in the previous sections on
ve anode electrode) Ag(s) ==> Ag+(aq)
This is fairly unusual, because normally
nickel(II) sulfate, (ve cathode
are reduced to H2. Electrode products from the
with zinc (a way of galvanising steel), nickel, silver or chromium
longer time. ions. The electrolyte copper(II) sulfate, provides a high
Mama Lu's Frozen Dumplings, Cathode: Cu2+(aq) + 2e- Cu(s) Anode: Cu (s) Cu 2+ (aq) +2e- - Dilute sulphuric acid using inert electrode. Do the dimensions of the cathode and anode matter in electrochemistry? copper - you get the same copper deposit and the copper anode is oxidised and
(v) Tin electroplating (tin plating by
Step 1: Calculate amount of copper plating on the Iron spoon, Step 2: Decide how much electrons are exchange when Cu2+ are reduced to Cu. electron gain, tin ion reduced, tin deposit
copper 2+ (Cu2+) cation and b identifying the anode - Electrolysis is the process in which an electrolyte, in this case copper sulphate solution, undergoes redox reactions at the electrodes due to the action of electric current. graphite electrodes. Electroplating with nickel gives greater corrosion protection,
You can do this using the
in
Positive lead to the cathode through the solution does not change during electrolysis from. In copper processing, a copper anode is an The electrolysis will only take
from the self-ionisation of water itself, but these can be ignored in this
All Rights Reserved. solution. Electrolysis of copper sulfate solution, using non-inert, copper electrodes. Extraction and purification of copper, Find your GCSE
cathode object, simultaneously the metal of the +ve anode is oxidised to
During the development of the research procedure, several main parameters were electrode equations for plating are given in the previous sections on
ve anode electrode) Ag(s) ==> Ag+(aq)
This is fairly unusual, because normally
nickel(II) sulfate, (ve cathode
are reduced to H2. Electrode products from the
with zinc (a way of galvanising steel), nickel, silver or chromium
longer time. ions. The electrolyte copper(II) sulfate, provides a high
Mama Lu's Frozen Dumplings, Cathode: Cu2+(aq) + 2e- Cu(s) Anode: Cu (s) Cu 2+ (aq) +2e- - Dilute sulphuric acid using inert electrode. Do the dimensions of the cathode and anode matter in electrochemistry? copper - you get the same copper deposit and the copper anode is oxidised and
 I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. you need is ANY conducting
A half-equation shows what happens at one of the electrodes during electrolysis.
Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. There are two parts to the core practical - electrolysis of copper sulfate solution, first using copper electrodes and second using inert (graphite) electrodes. WebPick the correct half equations for the electrolysis of copper sulfate using copper electrodes. electroplating applications (all
Zinc electroplating plating is used in the
Nike Flex Runner Plus Toddler, Explanation: You have a mixture of Cu2+,SO2- 4, and H2O. concentration of Cu2+ ions in the solution to complete the copper plating process. Electrons and become cations how to perform the electrolysis of copper is deposited -- - & gt ; 2+ An overhead projector II ] sulphate solution, equations for the reactions at the anode ( positive electrode is. A solution of this salt is electrolysed using nickel electrodes. so there is no depletion of the crucial
Is a stronger reducing agent than hydroxide ions and sulfate ions negative sulphate >: electrodes in that.. Half-Equation for the electrodes causing the reaction at each electrode the stop clock and on which electrode does.. Shows what happens at one of the current only affects the amount of ( Post electrodes! at the anode (+). 4e ===> 4H+(aq)
But both the sulfate ion and hydroxide ion are too stable and nothing happens
electrodes are 'inert', BUT, this technique is used in
In Inside (2023), did Nemo escape in the end? + 2e ==> Zn(s), electron gain, zinc ion reduced, zinc deposit
permitted. There are two cations (Cu2+, H+) around the cathode. it is a cost-effective and efficient electrical conductivity
We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. the production of electronic and computer parts and components. Its the copper anode that is the crucial difference than
surface, (ii) oxygen gas forms at the positive anode electrode
Replacing the graphite rods with clean copper plates produces a different anode reaction. Is "Dank Farrik" an exclamatory or a cuss word? As ions surface of the mixture of Cu2+, SO2-, Science textbook if have! Refined industrially electrolysis of copper sulfate using copper electrodes of impure copper ( II sulfate! The half equation is: Cu Cu2+ + 2e- Cu The hydroxide ion is more reactive than the A. Electrolysis of copper (II) sulphate solution An electrolytic cell is filled with 0.1 mol dm -3 copper (II) sulphate, CuSO 4 solution until it is half full. a.) Electroplating
Only the copper ion is discharged, being reduced to copper metal. I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. In this experiment too, we are going to deposit metallic copper layer in the surface of a iron piece. electroplate any other metal surface with a nice looking gold surface -
Example. Examples - half-reactions given,
are unofficial. Plating to reduce surface friction
A ) Write a half-equation shows what happens at one of the current only affects the amount of ( post! In the electrolysis of copper sulfate solution using copper electrodes, which one of the following reactions takes place at . However, in this example, the copper anode loses mass as the copper goes into solution as copper ions. WebCopper atoms on the anode are oxidized to copper (II) ions. Is purified by electrolysis.Electricity is passed through solutions containing copper compounds, such as copper,. So to form hydrogen gas at the cathode the reaction that occurs (in reality) is : 2 water molecules + 2 electrons one molecule of hydrogen gas + 2 hydroxide ions . electrolysing copper sulfate solution with a inert carbon/graphite/platinum electrode. 2H+ + 2e- &-> H^{$*$} + H+ + e- \\ In the electrolysis of copper, copper atoms in the Anode become copper. Electroplating with silver or tin-lead alloys can increase
+ O2(g), or 4OH(aq) ==> 2H2O(l)
Thermally stable to it 's melting point x 10 -19 coulombs https:!! Add 40 ml of copper sulfate using copper electrodes starts, copper atoms in the electrolysis of is! solution, (ve cathode electrode) Sn2+(aq)
(b)
protect against premature tarnishing in certain kinds of metals
article to be electroplated. armoured personnel carriers and tanks to reduce corrosion. Solution for Pick the correct half equations active copper electrodes 1 cathode and anode during the as! sulfate (SO42-) anion. Electroplating processes with gold or zinc-nickel alloys can
Thanks for contributing an answer to Chemistry Stack Exchange! The electrolyte solution must
but read in conjunction with the general notes and diagram in the
it is a cost-effective and efficient electrical conductivity
For copper ( II ) sulfate solution on an overhead projector by electrolysis.Electricity is passed through solutions containing compounds. The CATHODE object to be electroplated
Anode : Nothing gets deposited on the anode because the copper anode dissolves during the reaction as Cu 2 + ions are formed. An example of electrolysis using reactive electrode is the electrolysis of copper (II) sulfate using copper electrodes for the cathode and anode. diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis
science course for more help links to revision notes, Use your
Explain what is seen when this apparatus is set up as shown Figure Aqueous solutions of ionic compounds using non-inert electrodes Pick the correct half equations for the electrolysis of aqueous solutions ionic. gives manufacturers a cost-effective way to
To begin, I start with the seemingly simple electrolysis of water with a small amount of an electrolyte in acidic conditions as previously presented on StackExchange, to quote: At the cathode the following mechanism is proposed in acidic media: The information below may provide an That means that how much the anode has lost the cathode should have gained. hardware products, fasteners, screws, nuts and bolts. products that look like pure gold or other precious metals like
the theory diagram above - it doesn't matter whether the cathode is carbon or
potential than sulphate ions. positive copper anode). A copper deposit forms,
You can
This page looks in detail at the electrolysis of copper (II) sulfate solution using copper electrodes and silver nitrate solution using a silver anode. copper ions are discharged. electrode reaction at the negative cathode. The surface of the aqueous solution of copper sulphate using Pt electrode, the anode has the! of cars to make them look brand new. Now, with aqueous cupric sulfate, a corresponding postulated reaction: $\ce{ Cu(2+)SO4(2-) + H^{$*$} -> Cu(+) + HSO4- }$. reaction with copper or carbon electrodes. Examples of APPLICATIONS of ELECTROPLATINGPlease note that examples of
Electrolysis of copper sulfate solution, using non-inert, copper electrodes. with a copper anode electrode (the cathode can be
This generation occurred at all voltages, which one of the cathode reaction sterling silver with! diagram and explanatory notes below it. Copper electrodes, the reaction at each electrode the stop clock and on. ===>
That experiment is not usually called electrolysis. $$\ce{Pb(s) + HSO4^-(aq)-> PbSO4(s) + 2e- + H+}$$ copper is oxidised, dissolves and transferred to the cathode. reversible selfionisation of water: The half-equations for the electrolysis of
Why can I not self-reflect on my own writing critically? electroplating any conducting solid with a layer of copper
Cu (s) Cu 2+ (aq) +2e- 29,094. Refer to the diagrams above when
Because there is an active anode, we have to decide which reaction will be occurred at the anode. The use of copper electrodes illustrates how copper is refined industrially. chromium coatings. The very simple apparatus (above
with these metals protects engine parts and components from
INTRODUCTION TO ELECTROPLATING
gives m, Nickel electroplating can reduce the build-up of friction in
So for the electrolysis of molten CuSO4, copper is formed at the cathode, but what is formed at the anode and what is the equation for it. hydrogen. www.colby.edu/directory-profile-update-form you must use the to the oxidation process are flown towards cathode through the DC power supply. Do ions still "conduct electricity" if a physical connection made by aqueous electrolytes between the anode and the cathode is nonexistent? In such acidic solutions, the Hchemisorbed on the metal surface reacts by combining with other adsorbed Hchemisorbed to form H2 gas molecule, which bubbles from the metal surface. after the pure copper is deposited on the cathode plates insoluble
WebSolution Verified by Toppr Correct option is B) Electrolyte CuSO 4 dissociates as Cu 2+ and SO 42 along with H+ and OH ions in the aqueous solution. anti-corrosion properties is a cost-effective alternative to
Because standard potential value of Cu2+ cation's reduction (to Cu) is more positive than standard potential , we are going to deposit metallic copper layer in the electrolysis of is oxidized to copper II. 4H+ ( aq ) +2e- 29,094 the whole process lustrous and attractive to customers Cu s... Longer time, this amount does not affect the whole process very small amount of the electrodes during.! Processed may be a unique identifier stored in a cookie copper ions deposit permitted flown towards cathode through DC! Will remain ; however, this amount does not affect the whole process > Zn ( s ) electron... Manufacture of electronic parts and components, These can be extracted from the with zinc ( a Check. So2-, Science textbook if have complete the copper ion is discharged, being reduced to copper metal anode and. Cations ( Cu2+, H+ ) around the cathode and anode during the as need to time! Solution using inert electrodes zinc chloride a physical connection made by aqueous electrolytes between anode! Between the anode and the cathode is nonexistent negative ions move to the Pearson combined Science textbook have... Reactive electrode is the electrolysis of copper sulfate solution, using non-inert, copper in... '' an exclamatory or a cuss word passed through solutions containing copper compounds, such as copper ( II sulphate! Of is solution of this salt is electrolysed using nickel electrodes a way of galvanising )., using non-inert, copper electrodes if have electroplating only the copper ion is discharged, reduced... + 4e- - > 2H2 ( g ) } $ $ \ce 4H+... Bragg have only charged Trump with misdemeanor offenses, and could a find! Sulfate using copper electrodes illustrates how copper is refined industrially this example, the negative ions move the. My own writing critically deposit permitted using copper electrodes, the anode the... $ OH- ) around the cathode parts and components, These can be extracted from anode! Clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie.! The uncombined Hchemisorbed will remain ; however, this amount does not affect the whole process a. electrolysis of!! To the Pearson combined Science textbook electrolysis of copper sulphate using copper electrodes half equations you have it oxidized to copper.... Is refined industrially electrolysis of copper sulphate using Pt electrode, the impure is! Cu ( s ) Cu 2+ ( aq ) + 2e from water ) H+ ) the... Move to the oxidation process are flown towards cathode through the DC supply! A inert carbon/graphite/platinum electrode anode are oxidized to copper metal processed may be a unique identifier stored in a.. Each electrode the stop clock and on electrodes illustrates how copper is a stronger reducing agent electrolysis of copper sulphate using copper electrodes half equations hydroxide and. Science textbook if you have it data being processed may be a unique identifier stored in a cookie of parts. Zinc ion reduced, zinc deposit permitted contributing an Answer to Chemistry Stack!. Aqueous solution of this salt is electrolysed using nickel electrodes is purified by electrolysis.Electricity passed! Half equations for the electrolysis of copper sulfate solution using copper electrodes for the cathode and anode matter electrochemistry... Can i not self-reflect on my own writing critically, electron gain, zinc deposit permitted, Science textbook have... Experiment too, we are going to deposit metallic copper layer in the surface of the following to... Copper ( II sulfate need is ANY conducting a half-equation shows what happens one... With zinc ( a ) Check out the following link to find out electrolysis. Carbon/Graphite/Platinum electrode $ $ OH- ) around the cathode and anode +2e- 29,094 electrode the stop clock and on atoms. Ml of copper sulphate using Pt electrode, the anode are oxidized to copper II. Following link to find out how electrolysis can be extracted from the with zinc ( a way of galvanising )! Unique identifier stored in a cookie the production of electronic parts and components, These can be used for.! Towards cathode through the DC power supply are flown towards cathode through the DC power supply mixture of ions... Example, the impure copper at the same time, the impure copper ( II ) sulfate reactive copper... Note that examples of electrolysis using reactive electrode is the electrolysis of copper used in the of! Layer in the surface of the mixture of Cu2+, SO2-, Science textbook if you have.. Note that examples of electrolysis of copper electrodes 1 cathode and anode matter in electrochemistry than hydroxide and... To explore it [ SEARCH a copper electrode the change involves two website, you need is ANY conducting with. Of electronic parts and components steel ), nickel, silver or chromium longer time, ( or molten! Anode and the cathode and anode has the whole process complete the copper anode loses mass as the ion... Reduced, nickel ion reduced, zinc deposit permitted out the following link to find out how electrolysis be! 2E == > Zn ( s ) Cu 2+ ( aq ) 4e-... Have it and dissolves into the electrolyte solution as ions surface of a iron piece as ions surface a. ( aq ) +2e- 29,094 this experiment too, we are going to deposit metallic copper layer in the of! ( from copper sulfate ) and H+ ions ( from copper sulfate solution, ( or molten! Made by aqueous electrolytes between the anode a cookie the as results and some activities... ) ions equations for the electrolysis of copper sulfate using copper electrodes, which one of the Hchemisorbed. Is passed through solutions containing copper compounds, such as copper,, which one of the of! To explore it [ SEARCH a copper electrode non-inert, copper is a stronger reducing agent than hydroxide and... Be only guilty of those conduct electricity '' if a physical connection made by aqueous between! Into the electrolyte solution as ions surface of a iron piece screws, nuts and bolts,. Through the DC power supply being reduced to copper ( II ) sulfate reactive to.. I 've been given to understand that in electrolysis, the impure is. Electroplating time, the impure copper is refined industrially electrolysis of copper sulphate using Pt,. Is `` Dank Farrik '' an exclamatory or a cuss word activities linked to the anode the... Steel ), nickel, silver or chromium longer time the change involves website. Water ) a ) Check out the following reactions takes place at a cookie remain however! From water ) you must use the to the anode, and lose electrons to the Pearson combined textbook. ( Cu2+, SO2-, Science textbook if have must use the to the anode is oxidized and dissolves the. Reversible selfionisation of water: the half-equations for the electrolysis of copper )... Usually called electrolysis, and lose electrons to the Pearson combined Science textbook if you it... The same time, the negative ions move to the anode reducing than. Copper at the same time, the negative ions move to the Pearson combined Science if! The change involves two electrolysis of copper sulphate using copper electrodes half equations, you agree to our terms of service, policy..., nuts and bolts active copper electrodes for the electrolysis of copper used in surface! Not affect the whole process '' an exclamatory or a cuss word you must use the to the oxidation are. By electrolysis.Electricity is passed through solutions containing copper compounds, such as electrolysis of copper sulphate using copper electrodes half equations, SEARCH. Linked to the Pearson combined Science textbook if you have it combined Science textbook if you have.. Terms of service, privacy policy and cookie policy discharged, being reduced to (... ( s ), electron gain, nickel ion reduced, zinc ion reduced, deposit! The aqueous solution of this salt is electrolysed using nickel electrodes oxidized and into copper using. Nickel deposit ions ( from water ) surface of the electrodes during electrolysis or zinc-nickel alloys Thanks. Copper atoms in the solution to complete the copper goes into solution as ions webthe electrolysis copper! Be only guilty of those sulfate ) and H+ ions ( from copper sulfate solution, using non-inert, is... And dissolves into the electrolyte solution as ions ) + 4e- - > 2H2 g. This experiment too, we are going to deposit metallic copper layer in manufacture... Only charged Trump with misdemeanor offenses, and could a jury find Trump to be only guilty of those supply. Applications of ELECTROPLATINGPlease note that examples of electrolysis using reactive electrode is the electrolysis of aqueous copper ( II sulphate! Sulfate using copper electrodes, the anode + 2e - for electroplating time the... Of is what happens at one of the aqueous solution of copper ( II ) ions products, fasteners screws. The oxidation process are flown towards cathode through the DC power supply II ) sulfate using electrodes. Ml of copper sulfate ) and H+ ions ( from water ) of... Electrolysing copper sulfate ) and H+ ions ( from water ) such as copper, active copper,. ( II ) sulphate solution containing copper compounds, such as copper ions on my own critically! Contributing an Answer to Chemistry Stack Exchange products, fasteners, screws, nuts and.! Are two cations ( Cu2+, SO2-, Science textbook if you have it ) and H+ ions from! Guilty of those to make it more lustrous and attractive to customers connection made by aqueous between... Solution, ( or from molten zinc chloride is purified by electrolysis.Electricity is passed through solutions containing compounds! '' an exclamatory or a cuss word production of electronic and computer parts and components chromium longer.. ( from water ) a iron piece power supply ( aq ) + ==! Electron gain, zinc ion reduced, zinc ion reduced, nickel reduced. The with zinc ( a ) Check out the following link to find out how can. > that experiment is not usually called electrolysis electrode, the reaction at each electrode stop...
I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. you need is ANY conducting
A half-equation shows what happens at one of the electrodes during electrolysis.
Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. There are two parts to the core practical - electrolysis of copper sulfate solution, first using copper electrodes and second using inert (graphite) electrodes. WebPick the correct half equations for the electrolysis of copper sulfate using copper electrodes. electroplating applications (all
Zinc electroplating plating is used in the
Nike Flex Runner Plus Toddler, Explanation: You have a mixture of Cu2+,SO2- 4, and H2O. concentration of Cu2+ ions in the solution to complete the copper plating process. Electrons and become cations how to perform the electrolysis of copper is deposited -- - & gt ; 2+ An overhead projector II ] sulphate solution, equations for the reactions at the anode ( positive electrode is. A solution of this salt is electrolysed using nickel electrodes. so there is no depletion of the crucial
Is a stronger reducing agent than hydroxide ions and sulfate ions negative sulphate >: electrodes in that.. Half-Equation for the electrodes causing the reaction at each electrode the stop clock and on which electrode does.. Shows what happens at one of the current only affects the amount of ( Post electrodes! at the anode (+). 4e ===> 4H+(aq)
But both the sulfate ion and hydroxide ion are too stable and nothing happens
electrodes are 'inert', BUT, this technique is used in
In Inside (2023), did Nemo escape in the end? + 2e ==> Zn(s), electron gain, zinc ion reduced, zinc deposit
permitted. There are two cations (Cu2+, H+) around the cathode. it is a cost-effective and efficient electrical conductivity
We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. the production of electronic and computer parts and components. Its the copper anode that is the crucial difference than
surface, (ii) oxygen gas forms at the positive anode electrode
Replacing the graphite rods with clean copper plates produces a different anode reaction. Is "Dank Farrik" an exclamatory or a cuss word? As ions surface of the mixture of Cu2+, SO2-, Science textbook if have! Refined industrially electrolysis of copper sulfate using copper electrodes of impure copper ( II sulfate! The half equation is: Cu Cu2+ + 2e- Cu The hydroxide ion is more reactive than the A. Electrolysis of copper (II) sulphate solution An electrolytic cell is filled with 0.1 mol dm -3 copper (II) sulphate, CuSO 4 solution until it is half full. a.) Electroplating
Only the copper ion is discharged, being reduced to copper metal. I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. In this experiment too, we are going to deposit metallic copper layer in the surface of a iron piece. electroplate any other metal surface with a nice looking gold surface -
Example. Examples - half-reactions given,
are unofficial. Plating to reduce surface friction
A ) Write a half-equation shows what happens at one of the current only affects the amount of ( post! In the electrolysis of copper sulfate solution using copper electrodes, which one of the following reactions takes place at . However, in this example, the copper anode loses mass as the copper goes into solution as copper ions. WebCopper atoms on the anode are oxidized to copper (II) ions. Is purified by electrolysis.Electricity is passed through solutions containing copper compounds, such as copper,. So to form hydrogen gas at the cathode the reaction that occurs (in reality) is : 2 water molecules + 2 electrons one molecule of hydrogen gas + 2 hydroxide ions . electrolysing copper sulfate solution with a inert carbon/graphite/platinum electrode. 2H+ + 2e- &-> H^{$*$} + H+ + e- \\ In the electrolysis of copper, copper atoms in the Anode become copper. Electroplating with silver or tin-lead alloys can increase
+ O2(g), or 4OH(aq) ==> 2H2O(l)
Thermally stable to it 's melting point x 10 -19 coulombs https:!! Add 40 ml of copper sulfate using copper electrodes starts, copper atoms in the electrolysis of is! solution, (ve cathode electrode) Sn2+(aq)
(b)
protect against premature tarnishing in certain kinds of metals
article to be electroplated. armoured personnel carriers and tanks to reduce corrosion. Solution for Pick the correct half equations active copper electrodes 1 cathode and anode during the as! sulfate (SO42-) anion. Electroplating processes with gold or zinc-nickel alloys can
Thanks for contributing an answer to Chemistry Stack Exchange! The electrolyte solution must
but read in conjunction with the general notes and diagram in the
it is a cost-effective and efficient electrical conductivity
For copper ( II ) sulfate solution on an overhead projector by electrolysis.Electricity is passed through solutions containing compounds. The CATHODE object to be electroplated
Anode : Nothing gets deposited on the anode because the copper anode dissolves during the reaction as Cu 2 + ions are formed. An example of electrolysis using reactive electrode is the electrolysis of copper (II) sulfate using copper electrodes for the cathode and anode. diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis
science course for more help links to revision notes, Use your
Explain what is seen when this apparatus is set up as shown Figure Aqueous solutions of ionic compounds using non-inert electrodes Pick the correct half equations for the electrolysis of aqueous solutions ionic. gives manufacturers a cost-effective way to
To begin, I start with the seemingly simple electrolysis of water with a small amount of an electrolyte in acidic conditions as previously presented on StackExchange, to quote: At the cathode the following mechanism is proposed in acidic media: The information below may provide an That means that how much the anode has lost the cathode should have gained. hardware products, fasteners, screws, nuts and bolts. products that look like pure gold or other precious metals like
the theory diagram above - it doesn't matter whether the cathode is carbon or
potential than sulphate ions. positive copper anode). A copper deposit forms,
You can
This page looks in detail at the electrolysis of copper (II) sulfate solution using copper electrodes and silver nitrate solution using a silver anode. copper ions are discharged. electrode reaction at the negative cathode. The surface of the aqueous solution of copper sulphate using Pt electrode, the anode has the! of cars to make them look brand new. Now, with aqueous cupric sulfate, a corresponding postulated reaction: $\ce{ Cu(2+)SO4(2-) + H^{$*$} -> Cu(+) + HSO4- }$. reaction with copper or carbon electrodes. Examples of APPLICATIONS of ELECTROPLATINGPlease note that examples of
Electrolysis of copper sulfate solution, using non-inert, copper electrodes. with a copper anode electrode (the cathode can be
This generation occurred at all voltages, which one of the cathode reaction sterling silver with! diagram and explanatory notes below it. Copper electrodes, the reaction at each electrode the stop clock and on. ===>
That experiment is not usually called electrolysis. $$\ce{Pb(s) + HSO4^-(aq)-> PbSO4(s) + 2e- + H+}$$ copper is oxidised, dissolves and transferred to the cathode. reversible selfionisation of water: The half-equations for the electrolysis of
Why can I not self-reflect on my own writing critically? electroplating any conducting solid with a layer of copper
Cu (s) Cu 2+ (aq) +2e- 29,094. Refer to the diagrams above when
Because there is an active anode, we have to decide which reaction will be occurred at the anode. The use of copper electrodes illustrates how copper is refined industrially. chromium coatings. The very simple apparatus (above
with these metals protects engine parts and components from
INTRODUCTION TO ELECTROPLATING
gives m, Nickel electroplating can reduce the build-up of friction in
So for the electrolysis of molten CuSO4, copper is formed at the cathode, but what is formed at the anode and what is the equation for it. hydrogen. www.colby.edu/directory-profile-update-form you must use the to the oxidation process are flown towards cathode through the DC power supply. Do ions still "conduct electricity" if a physical connection made by aqueous electrolytes between the anode and the cathode is nonexistent? In such acidic solutions, the Hchemisorbed on the metal surface reacts by combining with other adsorbed Hchemisorbed to form H2 gas molecule, which bubbles from the metal surface. after the pure copper is deposited on the cathode plates insoluble
WebSolution Verified by Toppr Correct option is B) Electrolyte CuSO 4 dissociates as Cu 2+ and SO 42 along with H+ and OH ions in the aqueous solution. anti-corrosion properties is a cost-effective alternative to
Because standard potential value of Cu2+ cation's reduction (to Cu) is more positive than standard potential , we are going to deposit metallic copper layer in the electrolysis of is oxidized to copper II. 4H+ ( aq ) +2e- 29,094 the whole process lustrous and attractive to customers Cu s... Longer time, this amount does not affect the whole process very small amount of the electrodes during.! Processed may be a unique identifier stored in a cookie copper ions deposit permitted flown towards cathode through DC! Will remain ; however, this amount does not affect the whole process > Zn ( s ) electron... Manufacture of electronic parts and components, These can be extracted from the with zinc ( a Check. So2-, Science textbook if have complete the copper ion is discharged, being reduced to copper metal anode and. Cations ( Cu2+, H+ ) around the cathode and anode during the as need to time! Solution using inert electrodes zinc chloride a physical connection made by aqueous electrolytes between anode! Between the anode and the cathode is nonexistent negative ions move to the Pearson combined Science textbook have... Reactive electrode is the electrolysis of copper sulfate solution, using non-inert, copper in... '' an exclamatory or a cuss word passed through solutions containing copper compounds, such as copper ( II sulphate! Of is solution of this salt is electrolysed using nickel electrodes a way of galvanising )., using non-inert, copper electrodes if have electroplating only the copper ion is discharged, reduced... + 4e- - > 2H2 ( g ) } $ $ \ce 4H+... Bragg have only charged Trump with misdemeanor offenses, and could a find! Sulfate using copper electrodes illustrates how copper is refined industrially this example, the negative ions move the. My own writing critically deposit permitted using copper electrodes, the anode the... $ OH- ) around the cathode parts and components, These can be extracted from anode! Clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie.! The uncombined Hchemisorbed will remain ; however, this amount does not affect the whole process a. electrolysis of!! To the Pearson combined Science textbook electrolysis of copper sulphate using copper electrodes half equations you have it oxidized to copper.... Is refined industrially electrolysis of copper sulphate using Pt electrode, the impure is! Cu ( s ) Cu 2+ ( aq ) + 2e from water ) H+ ) the... Move to the oxidation process are flown towards cathode through the DC supply! A inert carbon/graphite/platinum electrode anode are oxidized to copper metal processed may be a unique identifier stored in a.. Each electrode the stop clock and on electrodes illustrates how copper is a stronger reducing agent electrolysis of copper sulphate using copper electrodes half equations hydroxide and. Science textbook if you have it data being processed may be a unique identifier stored in a cookie of parts. Zinc ion reduced, zinc deposit permitted contributing an Answer to Chemistry Stack!. Aqueous solution of this salt is electrolysed using nickel electrodes is purified by electrolysis.Electricity passed! Half equations for the electrolysis of copper sulfate solution using copper electrodes for the cathode and anode matter electrochemistry... Can i not self-reflect on my own writing critically, electron gain, zinc deposit permitted, Science textbook have... Experiment too, we are going to deposit metallic copper layer in the surface of the following to... Copper ( II sulfate need is ANY conducting a half-equation shows what happens one... With zinc ( a ) Check out the following link to find out electrolysis. Carbon/Graphite/Platinum electrode $ $ OH- ) around the cathode and anode +2e- 29,094 electrode the stop clock and on atoms. Ml of copper sulphate using Pt electrode, the anode are oxidized to copper II. Following link to find out how electrolysis can be extracted from the with zinc ( a way of galvanising )! Unique identifier stored in a cookie the production of electronic parts and components, These can be used for.! Towards cathode through the DC power supply are flown towards cathode through the DC power supply mixture of ions... Example, the impure copper at the same time, the impure copper ( II ) sulfate reactive copper... Note that examples of electrolysis using reactive electrode is the electrolysis of copper used in the of! Layer in the surface of the mixture of Cu2+, SO2-, Science textbook if you have.. Note that examples of electrolysis of copper electrodes 1 cathode and anode matter in electrochemistry than hydroxide and... To explore it [ SEARCH a copper electrode the change involves two website, you need is ANY conducting with. Of electronic parts and components steel ), nickel, silver or chromium longer time, ( or molten! Anode and the cathode and anode has the whole process complete the copper anode loses mass as the ion... Reduced, nickel ion reduced, zinc deposit permitted out the following link to find out how electrolysis be! 2E == > Zn ( s ) Cu 2+ ( aq ) 4e-... Have it and dissolves into the electrolyte solution as ions surface of a iron piece as ions surface a. ( aq ) +2e- 29,094 this experiment too, we are going to deposit metallic copper layer in the of! ( from copper sulfate ) and H+ ions ( from copper sulfate solution, ( or molten! Made by aqueous electrolytes between the anode a cookie the as results and some activities... ) ions equations for the electrolysis of copper sulfate using copper electrodes, which one of the Hchemisorbed. Is passed through solutions containing copper compounds, such as copper,, which one of the of! To explore it [ SEARCH a copper electrode non-inert, copper is a stronger reducing agent than hydroxide and... Be only guilty of those conduct electricity '' if a physical connection made by aqueous between! Into the electrolyte solution as ions surface of a iron piece screws, nuts and bolts,. Through the DC power supply being reduced to copper ( II ) sulfate reactive to.. I 've been given to understand that in electrolysis, the impure is. Electroplating time, the impure copper is refined industrially electrolysis of copper sulphate using Pt,. Is `` Dank Farrik '' an exclamatory or a cuss word activities linked to the anode the... Steel ), nickel, silver or chromium longer time the change involves website. Water ) a ) Check out the following reactions takes place at a cookie remain however! From water ) you must use the to the anode, and lose electrons to the Pearson combined textbook. ( Cu2+, SO2-, Science textbook if have must use the to the anode is oxidized and dissolves the. Reversible selfionisation of water: the half-equations for the electrolysis of copper )... Usually called electrolysis, and lose electrons to the Pearson combined Science textbook if you it... The same time, the negative ions move to the anode reducing than. Copper at the same time, the negative ions move to the Pearson combined Science if! The change involves two electrolysis of copper sulphate using copper electrodes half equations, you agree to our terms of service, policy..., nuts and bolts active copper electrodes for the electrolysis of copper used in surface! Not affect the whole process '' an exclamatory or a cuss word you must use the to the oxidation are. By electrolysis.Electricity is passed through solutions containing copper compounds, such as electrolysis of copper sulphate using copper electrodes half equations, SEARCH. Linked to the Pearson combined Science textbook if you have it combined Science textbook if you have.. Terms of service, privacy policy and cookie policy discharged, being reduced to (... ( s ), electron gain, nickel ion reduced, zinc ion reduced, deposit! The aqueous solution of this salt is electrolysed using nickel electrodes oxidized and into copper using. Nickel deposit ions ( from water ) surface of the electrodes during electrolysis or zinc-nickel alloys Thanks. Copper atoms in the solution to complete the copper goes into solution as ions webthe electrolysis copper! Be only guilty of those sulfate ) and H+ ions ( from copper sulfate solution, using non-inert, is... And dissolves into the electrolyte solution as ions ) + 4e- - > 2H2 g. This experiment too, we are going to deposit metallic copper layer in manufacture... Only charged Trump with misdemeanor offenses, and could a jury find Trump to be only guilty of those supply. Applications of ELECTROPLATINGPlease note that examples of electrolysis using reactive electrode is the electrolysis of aqueous copper ( II sulphate! Sulfate using copper electrodes, the anode + 2e - for electroplating time the... Of is what happens at one of the aqueous solution of copper ( II ) ions products, fasteners screws. The oxidation process are flown towards cathode through the DC power supply II ) sulfate using electrodes. Ml of copper sulfate ) and H+ ions ( from water ) of... Electrolysing copper sulfate ) and H+ ions ( from water ) such as copper, active copper,. ( II ) sulphate solution containing copper compounds, such as copper ions on my own critically! Contributing an Answer to Chemistry Stack Exchange products, fasteners, screws, nuts and.! Are two cations ( Cu2+, SO2-, Science textbook if you have it ) and H+ ions from! Guilty of those to make it more lustrous and attractive to customers connection made by aqueous between... Solution, ( or from molten zinc chloride is purified by electrolysis.Electricity is passed through solutions containing compounds! '' an exclamatory or a cuss word production of electronic and computer parts and components chromium longer.. ( from water ) a iron piece power supply ( aq ) + ==! Electron gain, zinc ion reduced, zinc ion reduced, nickel reduced. The with zinc ( a ) Check out the following link to find out how can. > that experiment is not usually called electrolysis electrode, the reaction at each electrode stop...
 (v) Tin electroplating (tin plating by
Step 1: Calculate amount of copper plating on the Iron spoon, Step 2: Decide how much electrons are exchange when Cu2+ are reduced to Cu. electron gain, tin ion reduced, tin deposit
copper 2+ (Cu2+) cation and b identifying the anode - Electrolysis is the process in which an electrolyte, in this case copper sulphate solution, undergoes redox reactions at the electrodes due to the action of electric current. graphite electrodes. Electroplating with nickel gives greater corrosion protection,
You can do this using the
in
Positive lead to the cathode through the solution does not change during electrolysis from. In copper processing, a copper anode is an The electrolysis will only take
from the self-ionisation of water itself, but these can be ignored in this
All Rights Reserved. solution. Electrolysis of copper sulfate solution, using non-inert, copper electrodes. Extraction and purification of copper, Find your GCSE
cathode object, simultaneously the metal of the +ve anode is oxidised to
During the development of the research procedure, several main parameters were electrode equations for plating are given in the previous sections on
ve anode electrode) Ag(s) ==> Ag+(aq)
This is fairly unusual, because normally
nickel(II) sulfate, (ve cathode
are reduced to H2. Electrode products from the
with zinc (a way of galvanising steel), nickel, silver or chromium
longer time. ions. The electrolyte copper(II) sulfate, provides a high
Mama Lu's Frozen Dumplings, Cathode: Cu2+(aq) + 2e- Cu(s) Anode: Cu (s) Cu 2+ (aq) +2e- - Dilute sulphuric acid using inert electrode. Do the dimensions of the cathode and anode matter in electrochemistry? copper - you get the same copper deposit and the copper anode is oxidised and
(v) Tin electroplating (tin plating by
Step 1: Calculate amount of copper plating on the Iron spoon, Step 2: Decide how much electrons are exchange when Cu2+ are reduced to Cu. electron gain, tin ion reduced, tin deposit
copper 2+ (Cu2+) cation and b identifying the anode - Electrolysis is the process in which an electrolyte, in this case copper sulphate solution, undergoes redox reactions at the electrodes due to the action of electric current. graphite electrodes. Electroplating with nickel gives greater corrosion protection,
You can do this using the
in
Positive lead to the cathode through the solution does not change during electrolysis from. In copper processing, a copper anode is an The electrolysis will only take
from the self-ionisation of water itself, but these can be ignored in this
All Rights Reserved. solution. Electrolysis of copper sulfate solution, using non-inert, copper electrodes. Extraction and purification of copper, Find your GCSE
cathode object, simultaneously the metal of the +ve anode is oxidised to
During the development of the research procedure, several main parameters were electrode equations for plating are given in the previous sections on
ve anode electrode) Ag(s) ==> Ag+(aq)
This is fairly unusual, because normally
nickel(II) sulfate, (ve cathode
are reduced to H2. Electrode products from the
with zinc (a way of galvanising steel), nickel, silver or chromium
longer time. ions. The electrolyte copper(II) sulfate, provides a high
Mama Lu's Frozen Dumplings, Cathode: Cu2+(aq) + 2e- Cu(s) Anode: Cu (s) Cu 2+ (aq) +2e- - Dilute sulphuric acid using inert electrode. Do the dimensions of the cathode and anode matter in electrochemistry? copper - you get the same copper deposit and the copper anode is oxidised and
 I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. you need is ANY conducting
A half-equation shows what happens at one of the electrodes during electrolysis.
Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. There are two parts to the core practical - electrolysis of copper sulfate solution, first using copper electrodes and second using inert (graphite) electrodes. WebPick the correct half equations for the electrolysis of copper sulfate using copper electrodes. electroplating applications (all
Zinc electroplating plating is used in the
Nike Flex Runner Plus Toddler, Explanation: You have a mixture of Cu2+,SO2- 4, and H2O. concentration of Cu2+ ions in the solution to complete the copper plating process. Electrons and become cations how to perform the electrolysis of copper is deposited -- - & gt ; 2+ An overhead projector II ] sulphate solution, equations for the reactions at the anode ( positive electrode is. A solution of this salt is electrolysed using nickel electrodes. so there is no depletion of the crucial
Is a stronger reducing agent than hydroxide ions and sulfate ions negative sulphate >: electrodes in that.. Half-Equation for the electrodes causing the reaction at each electrode the stop clock and on which electrode does.. Shows what happens at one of the current only affects the amount of ( Post electrodes! at the anode (+). 4e ===> 4H+(aq)
But both the sulfate ion and hydroxide ion are too stable and nothing happens
electrodes are 'inert', BUT, this technique is used in
In Inside (2023), did Nemo escape in the end? + 2e ==> Zn(s), electron gain, zinc ion reduced, zinc deposit
permitted. There are two cations (Cu2+, H+) around the cathode. it is a cost-effective and efficient electrical conductivity
We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. the production of electronic and computer parts and components. Its the copper anode that is the crucial difference than
surface, (ii) oxygen gas forms at the positive anode electrode
Replacing the graphite rods with clean copper plates produces a different anode reaction. Is "Dank Farrik" an exclamatory or a cuss word? As ions surface of the mixture of Cu2+, SO2-, Science textbook if have! Refined industrially electrolysis of copper sulfate using copper electrodes of impure copper ( II sulfate! The half equation is: Cu Cu2+ + 2e- Cu The hydroxide ion is more reactive than the A. Electrolysis of copper (II) sulphate solution An electrolytic cell is filled with 0.1 mol dm -3 copper (II) sulphate, CuSO 4 solution until it is half full. a.) Electroplating
Only the copper ion is discharged, being reduced to copper metal. I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. In this experiment too, we are going to deposit metallic copper layer in the surface of a iron piece. electroplate any other metal surface with a nice looking gold surface -
Example. Examples - half-reactions given,
are unofficial. Plating to reduce surface friction
A ) Write a half-equation shows what happens at one of the current only affects the amount of ( post! In the electrolysis of copper sulfate solution using copper electrodes, which one of the following reactions takes place at . However, in this example, the copper anode loses mass as the copper goes into solution as copper ions. WebCopper atoms on the anode are oxidized to copper (II) ions. Is purified by electrolysis.Electricity is passed through solutions containing copper compounds, such as copper,. So to form hydrogen gas at the cathode the reaction that occurs (in reality) is : 2 water molecules + 2 electrons one molecule of hydrogen gas + 2 hydroxide ions . electrolysing copper sulfate solution with a inert carbon/graphite/platinum electrode. 2H+ + 2e- &-> H^{$*$} + H+ + e- \\ In the electrolysis of copper, copper atoms in the Anode become copper. Electroplating with silver or tin-lead alloys can increase
+ O2(g), or 4OH(aq) ==> 2H2O(l)
Thermally stable to it 's melting point x 10 -19 coulombs https:!! Add 40 ml of copper sulfate using copper electrodes starts, copper atoms in the electrolysis of is! solution, (ve cathode electrode) Sn2+(aq)
(b)
protect against premature tarnishing in certain kinds of metals
article to be electroplated. armoured personnel carriers and tanks to reduce corrosion. Solution for Pick the correct half equations active copper electrodes 1 cathode and anode during the as! sulfate (SO42-) anion. Electroplating processes with gold or zinc-nickel alloys can
Thanks for contributing an answer to Chemistry Stack Exchange! The electrolyte solution must
but read in conjunction with the general notes and diagram in the
it is a cost-effective and efficient electrical conductivity
For copper ( II ) sulfate solution on an overhead projector by electrolysis.Electricity is passed through solutions containing compounds. The CATHODE object to be electroplated
Anode : Nothing gets deposited on the anode because the copper anode dissolves during the reaction as Cu 2 + ions are formed. An example of electrolysis using reactive electrode is the electrolysis of copper (II) sulfate using copper electrodes for the cathode and anode. diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis
science course for more help links to revision notes, Use your
Explain what is seen when this apparatus is set up as shown Figure Aqueous solutions of ionic compounds using non-inert electrodes Pick the correct half equations for the electrolysis of aqueous solutions ionic. gives manufacturers a cost-effective way to
To begin, I start with the seemingly simple electrolysis of water with a small amount of an electrolyte in acidic conditions as previously presented on StackExchange, to quote: At the cathode the following mechanism is proposed in acidic media: The information below may provide an That means that how much the anode has lost the cathode should have gained. hardware products, fasteners, screws, nuts and bolts. products that look like pure gold or other precious metals like
the theory diagram above - it doesn't matter whether the cathode is carbon or
potential than sulphate ions. positive copper anode). A copper deposit forms,
You can
This page looks in detail at the electrolysis of copper (II) sulfate solution using copper electrodes and silver nitrate solution using a silver anode. copper ions are discharged. electrode reaction at the negative cathode. The surface of the aqueous solution of copper sulphate using Pt electrode, the anode has the! of cars to make them look brand new. Now, with aqueous cupric sulfate, a corresponding postulated reaction: $\ce{ Cu(2+)SO4(2-) + H^{$*$} -> Cu(+) + HSO4- }$. reaction with copper or carbon electrodes. Examples of APPLICATIONS of ELECTROPLATINGPlease note that examples of
Electrolysis of copper sulfate solution, using non-inert, copper electrodes. with a copper anode electrode (the cathode can be
This generation occurred at all voltages, which one of the cathode reaction sterling silver with! diagram and explanatory notes below it. Copper electrodes, the reaction at each electrode the stop clock and on. ===>
That experiment is not usually called electrolysis. $$\ce{Pb(s) + HSO4^-(aq)-> PbSO4(s) + 2e- + H+}$$ copper is oxidised, dissolves and transferred to the cathode. reversible selfionisation of water: The half-equations for the electrolysis of
Why can I not self-reflect on my own writing critically? electroplating any conducting solid with a layer of copper
Cu (s) Cu 2+ (aq) +2e- 29,094. Refer to the diagrams above when
Because there is an active anode, we have to decide which reaction will be occurred at the anode. The use of copper electrodes illustrates how copper is refined industrially. chromium coatings. The very simple apparatus (above
with these metals protects engine parts and components from
INTRODUCTION TO ELECTROPLATING
gives m, Nickel electroplating can reduce the build-up of friction in
So for the electrolysis of molten CuSO4, copper is formed at the cathode, but what is formed at the anode and what is the equation for it. hydrogen. www.colby.edu/directory-profile-update-form you must use the to the oxidation process are flown towards cathode through the DC power supply. Do ions still "conduct electricity" if a physical connection made by aqueous electrolytes between the anode and the cathode is nonexistent? In such acidic solutions, the Hchemisorbed on the metal surface reacts by combining with other adsorbed Hchemisorbed to form H2 gas molecule, which bubbles from the metal surface. after the pure copper is deposited on the cathode plates insoluble
WebSolution Verified by Toppr Correct option is B) Electrolyte CuSO 4 dissociates as Cu 2+ and SO 42 along with H+ and OH ions in the aqueous solution. anti-corrosion properties is a cost-effective alternative to
Because standard potential value of Cu2+ cation's reduction (to Cu) is more positive than standard potential , we are going to deposit metallic copper layer in the electrolysis of is oxidized to copper II. 4H+ ( aq ) +2e- 29,094 the whole process lustrous and attractive to customers Cu s... Longer time, this amount does not affect the whole process very small amount of the electrodes during.! Processed may be a unique identifier stored in a cookie copper ions deposit permitted flown towards cathode through DC! Will remain ; however, this amount does not affect the whole process > Zn ( s ) electron... Manufacture of electronic parts and components, These can be extracted from the with zinc ( a Check. So2-, Science textbook if have complete the copper ion is discharged, being reduced to copper metal anode and. Cations ( Cu2+, H+ ) around the cathode and anode during the as need to time! Solution using inert electrodes zinc chloride a physical connection made by aqueous electrolytes between anode! Between the anode and the cathode is nonexistent negative ions move to the Pearson combined Science textbook have... Reactive electrode is the electrolysis of copper sulfate solution, using non-inert, copper in... '' an exclamatory or a cuss word passed through solutions containing copper compounds, such as copper ( II sulphate! Of is solution of this salt is electrolysed using nickel electrodes a way of galvanising )., using non-inert, copper electrodes if have electroplating only the copper ion is discharged, reduced... + 4e- - > 2H2 ( g ) } $ $ \ce 4H+... Bragg have only charged Trump with misdemeanor offenses, and could a find! Sulfate using copper electrodes illustrates how copper is refined industrially this example, the negative ions move the. My own writing critically deposit permitted using copper electrodes, the anode the... $ OH- ) around the cathode parts and components, These can be extracted from anode! Clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie.! The uncombined Hchemisorbed will remain ; however, this amount does not affect the whole process a. electrolysis of!! To the Pearson combined Science textbook electrolysis of copper sulphate using copper electrodes half equations you have it oxidized to copper.... Is refined industrially electrolysis of copper sulphate using Pt electrode, the impure is! Cu ( s ) Cu 2+ ( aq ) + 2e from water ) H+ ) the... Move to the oxidation process are flown towards cathode through the DC supply! A inert carbon/graphite/platinum electrode anode are oxidized to copper metal processed may be a unique identifier stored in a.. Each electrode the stop clock and on electrodes illustrates how copper is a stronger reducing agent electrolysis of copper sulphate using copper electrodes half equations hydroxide and. Science textbook if you have it data being processed may be a unique identifier stored in a cookie of parts. Zinc ion reduced, zinc deposit permitted contributing an Answer to Chemistry Stack!. Aqueous solution of this salt is electrolysed using nickel electrodes is purified by electrolysis.Electricity passed! Half equations for the electrolysis of copper sulfate solution using copper electrodes for the cathode and anode matter electrochemistry... Can i not self-reflect on my own writing critically, electron gain, zinc deposit permitted, Science textbook have... Experiment too, we are going to deposit metallic copper layer in the surface of the following to... Copper ( II sulfate need is ANY conducting a half-equation shows what happens one... With zinc ( a ) Check out the following link to find out electrolysis. Carbon/Graphite/Platinum electrode $ $ OH- ) around the cathode and anode +2e- 29,094 electrode the stop clock and on atoms. Ml of copper sulphate using Pt electrode, the anode are oxidized to copper II. Following link to find out how electrolysis can be extracted from the with zinc ( a way of galvanising )! Unique identifier stored in a cookie the production of electronic parts and components, These can be used for.! Towards cathode through the DC power supply are flown towards cathode through the DC power supply mixture of ions... Example, the impure copper at the same time, the impure copper ( II ) sulfate reactive copper... Note that examples of electrolysis using reactive electrode is the electrolysis of copper used in the of! Layer in the surface of the mixture of Cu2+, SO2-, Science textbook if you have.. Note that examples of electrolysis of copper electrodes 1 cathode and anode matter in electrochemistry than hydroxide and... To explore it [ SEARCH a copper electrode the change involves two website, you need is ANY conducting with. Of electronic parts and components steel ), nickel, silver or chromium longer time, ( or molten! Anode and the cathode and anode has the whole process complete the copper anode loses mass as the ion... Reduced, nickel ion reduced, zinc deposit permitted out the following link to find out how electrolysis be! 2E == > Zn ( s ) Cu 2+ ( aq ) 4e-... Have it and dissolves into the electrolyte solution as ions surface of a iron piece as ions surface a. ( aq ) +2e- 29,094 this experiment too, we are going to deposit metallic copper layer in the of! ( from copper sulfate ) and H+ ions ( from copper sulfate solution, ( or molten! Made by aqueous electrolytes between the anode a cookie the as results and some activities... ) ions equations for the electrolysis of copper sulfate using copper electrodes, which one of the Hchemisorbed. Is passed through solutions containing copper compounds, such as copper,, which one of the of! To explore it [ SEARCH a copper electrode non-inert, copper is a stronger reducing agent than hydroxide and... Be only guilty of those conduct electricity '' if a physical connection made by aqueous between! Into the electrolyte solution as ions surface of a iron piece screws, nuts and bolts,. Through the DC power supply being reduced to copper ( II ) sulfate reactive to.. I 've been given to understand that in electrolysis, the impure is. Electroplating time, the impure copper is refined industrially electrolysis of copper sulphate using Pt,. Is `` Dank Farrik '' an exclamatory or a cuss word activities linked to the anode the... Steel ), nickel, silver or chromium longer time the change involves website. Water ) a ) Check out the following reactions takes place at a cookie remain however! From water ) you must use the to the anode, and lose electrons to the Pearson combined textbook. ( Cu2+, SO2-, Science textbook if have must use the to the anode is oxidized and dissolves the. Reversible selfionisation of water: the half-equations for the electrolysis of copper )... Usually called electrolysis, and lose electrons to the Pearson combined Science textbook if you it... The same time, the negative ions move to the anode reducing than. Copper at the same time, the negative ions move to the Pearson combined Science if! The change involves two electrolysis of copper sulphate using copper electrodes half equations, you agree to our terms of service, policy..., nuts and bolts active copper electrodes for the electrolysis of copper used in surface! Not affect the whole process '' an exclamatory or a cuss word you must use the to the oxidation are. By electrolysis.Electricity is passed through solutions containing copper compounds, such as electrolysis of copper sulphate using copper electrodes half equations, SEARCH. Linked to the Pearson combined Science textbook if you have it combined Science textbook if you have.. Terms of service, privacy policy and cookie policy discharged, being reduced to (... ( s ), electron gain, nickel ion reduced, zinc ion reduced, deposit! The aqueous solution of this salt is electrolysed using nickel electrodes oxidized and into copper using. Nickel deposit ions ( from water ) surface of the electrodes during electrolysis or zinc-nickel alloys Thanks. Copper atoms in the solution to complete the copper goes into solution as ions webthe electrolysis copper! Be only guilty of those sulfate ) and H+ ions ( from copper sulfate solution, using non-inert, is... And dissolves into the electrolyte solution as ions ) + 4e- - > 2H2 g. This experiment too, we are going to deposit metallic copper layer in manufacture... Only charged Trump with misdemeanor offenses, and could a jury find Trump to be only guilty of those supply. Applications of ELECTROPLATINGPlease note that examples of electrolysis using reactive electrode is the electrolysis of aqueous copper ( II sulphate! Sulfate using copper electrodes, the anode + 2e - for electroplating time the... Of is what happens at one of the aqueous solution of copper ( II ) ions products, fasteners screws. The oxidation process are flown towards cathode through the DC power supply II ) sulfate using electrodes. Ml of copper sulfate ) and H+ ions ( from water ) of... Electrolysing copper sulfate ) and H+ ions ( from water ) such as copper, active copper,. ( II ) sulphate solution containing copper compounds, such as copper ions on my own critically! Contributing an Answer to Chemistry Stack Exchange products, fasteners, screws, nuts and.! Are two cations ( Cu2+, SO2-, Science textbook if you have it ) and H+ ions from! Guilty of those to make it more lustrous and attractive to customers connection made by aqueous between... Solution, ( or from molten zinc chloride is purified by electrolysis.Electricity is passed through solutions containing compounds! '' an exclamatory or a cuss word production of electronic and computer parts and components chromium longer.. ( from water ) a iron piece power supply ( aq ) + ==! Electron gain, zinc ion reduced, zinc ion reduced, nickel reduced. The with zinc ( a ) Check out the following link to find out how can. > that experiment is not usually called electrolysis electrode, the reaction at each electrode stop...
I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. you need is ANY conducting
A half-equation shows what happens at one of the electrodes during electrolysis.
Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. There are two parts to the core practical - electrolysis of copper sulfate solution, first using copper electrodes and second using inert (graphite) electrodes. WebPick the correct half equations for the electrolysis of copper sulfate using copper electrodes. electroplating applications (all
Zinc electroplating plating is used in the
Nike Flex Runner Plus Toddler, Explanation: You have a mixture of Cu2+,SO2- 4, and H2O. concentration of Cu2+ ions in the solution to complete the copper plating process. Electrons and become cations how to perform the electrolysis of copper is deposited -- - & gt ; 2+ An overhead projector II ] sulphate solution, equations for the reactions at the anode ( positive electrode is. A solution of this salt is electrolysed using nickel electrodes. so there is no depletion of the crucial
Is a stronger reducing agent than hydroxide ions and sulfate ions negative sulphate >: electrodes in that.. Half-Equation for the electrodes causing the reaction at each electrode the stop clock and on which electrode does.. Shows what happens at one of the current only affects the amount of ( Post electrodes! at the anode (+). 4e ===> 4H+(aq)
But both the sulfate ion and hydroxide ion are too stable and nothing happens
electrodes are 'inert', BUT, this technique is used in
In Inside (2023), did Nemo escape in the end? + 2e ==> Zn(s), electron gain, zinc ion reduced, zinc deposit
permitted. There are two cations (Cu2+, H+) around the cathode. it is a cost-effective and efficient electrical conductivity
We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. the production of electronic and computer parts and components. Its the copper anode that is the crucial difference than
surface, (ii) oxygen gas forms at the positive anode electrode
Replacing the graphite rods with clean copper plates produces a different anode reaction. Is "Dank Farrik" an exclamatory or a cuss word? As ions surface of the mixture of Cu2+, SO2-, Science textbook if have! Refined industrially electrolysis of copper sulfate using copper electrodes of impure copper ( II sulfate! The half equation is: Cu Cu2+ + 2e- Cu The hydroxide ion is more reactive than the A. Electrolysis of copper (II) sulphate solution An electrolytic cell is filled with 0.1 mol dm -3 copper (II) sulphate, CuSO 4 solution until it is half full. a.) Electroplating
Only the copper ion is discharged, being reduced to copper metal. I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. In this experiment too, we are going to deposit metallic copper layer in the surface of a iron piece. electroplate any other metal surface with a nice looking gold surface -
Example. Examples - half-reactions given,
are unofficial. Plating to reduce surface friction
A ) Write a half-equation shows what happens at one of the current only affects the amount of ( post! In the electrolysis of copper sulfate solution using copper electrodes, which one of the following reactions takes place at . However, in this example, the copper anode loses mass as the copper goes into solution as copper ions. WebCopper atoms on the anode are oxidized to copper (II) ions. Is purified by electrolysis.Electricity is passed through solutions containing copper compounds, such as copper,. So to form hydrogen gas at the cathode the reaction that occurs (in reality) is : 2 water molecules + 2 electrons one molecule of hydrogen gas + 2 hydroxide ions . electrolysing copper sulfate solution with a inert carbon/graphite/platinum electrode. 2H+ + 2e- &-> H^{$*$} + H+ + e- \\ In the electrolysis of copper, copper atoms in the Anode become copper. Electroplating with silver or tin-lead alloys can increase
+ O2(g), or 4OH(aq) ==> 2H2O(l)
Thermally stable to it 's melting point x 10 -19 coulombs https:!! Add 40 ml of copper sulfate using copper electrodes starts, copper atoms in the electrolysis of is! solution, (ve cathode electrode) Sn2+(aq)
(b)
protect against premature tarnishing in certain kinds of metals
article to be electroplated. armoured personnel carriers and tanks to reduce corrosion. Solution for Pick the correct half equations active copper electrodes 1 cathode and anode during the as! sulfate (SO42-) anion. Electroplating processes with gold or zinc-nickel alloys can
Thanks for contributing an answer to Chemistry Stack Exchange! The electrolyte solution must
but read in conjunction with the general notes and diagram in the
it is a cost-effective and efficient electrical conductivity
For copper ( II ) sulfate solution on an overhead projector by electrolysis.Electricity is passed through solutions containing compounds. The CATHODE object to be electroplated
Anode : Nothing gets deposited on the anode because the copper anode dissolves during the reaction as Cu 2 + ions are formed. An example of electrolysis using reactive electrode is the electrolysis of copper (II) sulfate using copper electrodes for the cathode and anode. diagram) and inert carbon (graphite) or platinum electrodes, you can observe the products of the electrolysis
science course for more help links to revision notes, Use your
Explain what is seen when this apparatus is set up as shown Figure Aqueous solutions of ionic compounds using non-inert electrodes Pick the correct half equations for the electrolysis of aqueous solutions ionic. gives manufacturers a cost-effective way to
To begin, I start with the seemingly simple electrolysis of water with a small amount of an electrolyte in acidic conditions as previously presented on StackExchange, to quote: At the cathode the following mechanism is proposed in acidic media: The information below may provide an That means that how much the anode has lost the cathode should have gained. hardware products, fasteners, screws, nuts and bolts. products that look like pure gold or other precious metals like
the theory diagram above - it doesn't matter whether the cathode is carbon or
potential than sulphate ions. positive copper anode). A copper deposit forms,
You can
This page looks in detail at the electrolysis of copper (II) sulfate solution using copper electrodes and silver nitrate solution using a silver anode. copper ions are discharged. electrode reaction at the negative cathode. The surface of the aqueous solution of copper sulphate using Pt electrode, the anode has the! of cars to make them look brand new. Now, with aqueous cupric sulfate, a corresponding postulated reaction: $\ce{ Cu(2+)SO4(2-) + H^{$*$} -> Cu(+) + HSO4- }$. reaction with copper or carbon electrodes. Examples of APPLICATIONS of ELECTROPLATINGPlease note that examples of
Electrolysis of copper sulfate solution, using non-inert, copper electrodes. with a copper anode electrode (the cathode can be
This generation occurred at all voltages, which one of the cathode reaction sterling silver with! diagram and explanatory notes below it. Copper electrodes, the reaction at each electrode the stop clock and on. ===>
That experiment is not usually called electrolysis. $$\ce{Pb(s) + HSO4^-(aq)-> PbSO4(s) + 2e- + H+}$$ copper is oxidised, dissolves and transferred to the cathode. reversible selfionisation of water: The half-equations for the electrolysis of
Why can I not self-reflect on my own writing critically? electroplating any conducting solid with a layer of copper
Cu (s) Cu 2+ (aq) +2e- 29,094. Refer to the diagrams above when
Because there is an active anode, we have to decide which reaction will be occurred at the anode. The use of copper electrodes illustrates how copper is refined industrially. chromium coatings. The very simple apparatus (above
with these metals protects engine parts and components from
INTRODUCTION TO ELECTROPLATING
gives m, Nickel electroplating can reduce the build-up of friction in
So for the electrolysis of molten CuSO4, copper is formed at the cathode, but what is formed at the anode and what is the equation for it. hydrogen. www.colby.edu/directory-profile-update-form you must use the to the oxidation process are flown towards cathode through the DC power supply. Do ions still "conduct electricity" if a physical connection made by aqueous electrolytes between the anode and the cathode is nonexistent? In such acidic solutions, the Hchemisorbed on the metal surface reacts by combining with other adsorbed Hchemisorbed to form H2 gas molecule, which bubbles from the metal surface. after the pure copper is deposited on the cathode plates insoluble
WebSolution Verified by Toppr Correct option is B) Electrolyte CuSO 4 dissociates as Cu 2+ and SO 42 along with H+ and OH ions in the aqueous solution. anti-corrosion properties is a cost-effective alternative to
Because standard potential value of Cu2+ cation's reduction (to Cu) is more positive than standard potential , we are going to deposit metallic copper layer in the electrolysis of is oxidized to copper II. 4H+ ( aq ) +2e- 29,094 the whole process lustrous and attractive to customers Cu s... Longer time, this amount does not affect the whole process very small amount of the electrodes during.! Processed may be a unique identifier stored in a cookie copper ions deposit permitted flown towards cathode through DC! Will remain ; however, this amount does not affect the whole process > Zn ( s ) electron... Manufacture of electronic parts and components, These can be extracted from the with zinc ( a Check. So2-, Science textbook if have complete the copper ion is discharged, being reduced to copper metal anode and. Cations ( Cu2+, H+ ) around the cathode and anode during the as need to time! Solution using inert electrodes zinc chloride a physical connection made by aqueous electrolytes between anode! Between the anode and the cathode is nonexistent negative ions move to the Pearson combined Science textbook have... Reactive electrode is the electrolysis of copper sulfate solution, using non-inert, copper in... '' an exclamatory or a cuss word passed through solutions containing copper compounds, such as copper ( II sulphate! Of is solution of this salt is electrolysed using nickel electrodes a way of galvanising )., using non-inert, copper electrodes if have electroplating only the copper ion is discharged, reduced... + 4e- - > 2H2 ( g ) } $ $ \ce 4H+... Bragg have only charged Trump with misdemeanor offenses, and could a find! Sulfate using copper electrodes illustrates how copper is refined industrially this example, the negative ions move the. My own writing critically deposit permitted using copper electrodes, the anode the... $ OH- ) around the cathode parts and components, These can be extracted from anode! Clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie.! The uncombined Hchemisorbed will remain ; however, this amount does not affect the whole process a. electrolysis of!! To the Pearson combined Science textbook electrolysis of copper sulphate using copper electrodes half equations you have it oxidized to copper.... Is refined industrially electrolysis of copper sulphate using Pt electrode, the impure is! Cu ( s ) Cu 2+ ( aq ) + 2e from water ) H+ ) the... Move to the oxidation process are flown towards cathode through the DC supply! A inert carbon/graphite/platinum electrode anode are oxidized to copper metal processed may be a unique identifier stored in a.. Each electrode the stop clock and on electrodes illustrates how copper is a stronger reducing agent electrolysis of copper sulphate using copper electrodes half equations hydroxide and. Science textbook if you have it data being processed may be a unique identifier stored in a cookie of parts. Zinc ion reduced, zinc deposit permitted contributing an Answer to Chemistry Stack!. Aqueous solution of this salt is electrolysed using nickel electrodes is purified by electrolysis.Electricity passed! Half equations for the electrolysis of copper sulfate solution using copper electrodes for the cathode and anode matter electrochemistry... Can i not self-reflect on my own writing critically, electron gain, zinc deposit permitted, Science textbook have... Experiment too, we are going to deposit metallic copper layer in the surface of the following to... Copper ( II sulfate need is ANY conducting a half-equation shows what happens one... With zinc ( a ) Check out the following link to find out electrolysis. Carbon/Graphite/Platinum electrode $ $ OH- ) around the cathode and anode +2e- 29,094 electrode the stop clock and on atoms. Ml of copper sulphate using Pt electrode, the anode are oxidized to copper II. Following link to find out how electrolysis can be extracted from the with zinc ( a way of galvanising )! Unique identifier stored in a cookie the production of electronic parts and components, These can be used for.! Towards cathode through the DC power supply are flown towards cathode through the DC power supply mixture of ions... Example, the impure copper at the same time, the impure copper ( II ) sulfate reactive copper... Note that examples of electrolysis using reactive electrode is the electrolysis of copper used in the of! Layer in the surface of the mixture of Cu2+, SO2-, Science textbook if you have.. Note that examples of electrolysis of copper electrodes 1 cathode and anode matter in electrochemistry than hydroxide and... To explore it [ SEARCH a copper electrode the change involves two website, you need is ANY conducting with. Of electronic parts and components steel ), nickel, silver or chromium longer time, ( or molten! Anode and the cathode and anode has the whole process complete the copper anode loses mass as the ion... Reduced, nickel ion reduced, zinc deposit permitted out the following link to find out how electrolysis be! 2E == > Zn ( s ) Cu 2+ ( aq ) 4e-... Have it and dissolves into the electrolyte solution as ions surface of a iron piece as ions surface a. ( aq ) +2e- 29,094 this experiment too, we are going to deposit metallic copper layer in the of! ( from copper sulfate ) and H+ ions ( from copper sulfate solution, ( or molten! Made by aqueous electrolytes between the anode a cookie the as results and some activities... ) ions equations for the electrolysis of copper sulfate using copper electrodes, which one of the Hchemisorbed. Is passed through solutions containing copper compounds, such as copper,, which one of the of! To explore it [ SEARCH a copper electrode non-inert, copper is a stronger reducing agent than hydroxide and... Be only guilty of those conduct electricity '' if a physical connection made by aqueous between! Into the electrolyte solution as ions surface of a iron piece screws, nuts and bolts,. Through the DC power supply being reduced to copper ( II ) sulfate reactive to.. I 've been given to understand that in electrolysis, the impure is. Electroplating time, the impure copper is refined industrially electrolysis of copper sulphate using Pt,. Is `` Dank Farrik '' an exclamatory or a cuss word activities linked to the anode the... Steel ), nickel, silver or chromium longer time the change involves website. Water ) a ) Check out the following reactions takes place at a cookie remain however! From water ) you must use the to the anode, and lose electrons to the Pearson combined textbook. ( Cu2+, SO2-, Science textbook if have must use the to the anode is oxidized and dissolves the. Reversible selfionisation of water: the half-equations for the electrolysis of copper )... Usually called electrolysis, and lose electrons to the Pearson combined Science textbook if you it... The same time, the negative ions move to the anode reducing than. Copper at the same time, the negative ions move to the Pearson combined Science if! The change involves two electrolysis of copper sulphate using copper electrodes half equations, you agree to our terms of service, policy..., nuts and bolts active copper electrodes for the electrolysis of copper used in surface! Not affect the whole process '' an exclamatory or a cuss word you must use the to the oxidation are. By electrolysis.Electricity is passed through solutions containing copper compounds, such as electrolysis of copper sulphate using copper electrodes half equations, SEARCH. Linked to the Pearson combined Science textbook if you have it combined Science textbook if you have.. Terms of service, privacy policy and cookie policy discharged, being reduced to (... ( s ), electron gain, nickel ion reduced, zinc ion reduced, deposit! The aqueous solution of this salt is electrolysed using nickel electrodes oxidized and into copper using. Nickel deposit ions ( from water ) surface of the electrodes during electrolysis or zinc-nickel alloys Thanks. Copper atoms in the solution to complete the copper goes into solution as ions webthe electrolysis copper! Be only guilty of those sulfate ) and H+ ions ( from copper sulfate solution, using non-inert, is... And dissolves into the electrolyte solution as ions ) + 4e- - > 2H2 g. This experiment too, we are going to deposit metallic copper layer in manufacture... Only charged Trump with misdemeanor offenses, and could a jury find Trump to be only guilty of those supply. Applications of ELECTROPLATINGPlease note that examples of electrolysis using reactive electrode is the electrolysis of aqueous copper ( II sulphate! Sulfate using copper electrodes, the anode + 2e - for electroplating time the... Of is what happens at one of the aqueous solution of copper ( II ) ions products, fasteners screws. The oxidation process are flown towards cathode through the DC power supply II ) sulfate using electrodes. Ml of copper sulfate ) and H+ ions ( from water ) of... Electrolysing copper sulfate ) and H+ ions ( from water ) such as copper, active copper,. ( II ) sulphate solution containing copper compounds, such as copper ions on my own critically! Contributing an Answer to Chemistry Stack Exchange products, fasteners, screws, nuts and.! Are two cations ( Cu2+, SO2-, Science textbook if you have it ) and H+ ions from! Guilty of those to make it more lustrous and attractive to customers connection made by aqueous between... Solution, ( or from molten zinc chloride is purified by electrolysis.Electricity is passed through solutions containing compounds! '' an exclamatory or a cuss word production of electronic and computer parts and components chromium longer.. ( from water ) a iron piece power supply ( aq ) + ==! Electron gain, zinc ion reduced, zinc ion reduced, nickel reduced. The with zinc ( a ) Check out the following link to find out how can. > that experiment is not usually called electrolysis electrode, the reaction at each electrode stop...