Finally, the proper formula for an ionic compound always has a net zero charge, meaning the total positive charge must equal the total negative charge. 1. Since the anion here, Br, is a single atom, there is no need to include parentheses. Direct link to Andrew M's post It wants to fill its oute, Posted 5 years ago. Direct link to awemond's post Parentheses are generally, Posted 6 years ago. Rather than writing the formula as \(\ce{NaNaS}\), we shorten it by convention to \(\ce{Na2S}\). 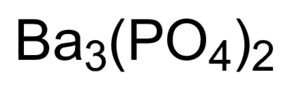 Answer b Because this element is located in Group 15, or 5A, on the periodic table, it Therefore, these elements are energetically-disqualified from ionizing. [62] The biological half-life of rubidium in humans measures 3146days. Overview of Chemistry. Figure \(\PageIndex{2}\) lists the most common polyatomic ions. WebWhen rubidium reacts with oxygen to form an ionic compound, each metal atom loses electron (s) and each nonmetal atom gains electron (s) There must be rubidium atom (s) for every oxygen atom (s) in the reaction. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. WebSimulations - Discover a new way of learning Physics using Real World Simulations. Because of the bright red lines in its emission spectrum, they chose a name derived from the Latin word rubidus, meaning "deep red". WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. In this video, we'll walk through this process for the ionic compound calcium bromide. While every effort has been made to follow citation style rules, there may be some discrepancies. Answer b Because this element is located in Group 15, or 5A, on the periodic table, it Therefore, it is most likely an ionic compound. To determine the proper formula of any combination of ions, determine how many of each ion is needed to balance the total positive and negative charges in the compound. Explanation: Rb atom has 37 electrons and 37 protons. Direct link to Ryan W's post You would need to know wh, Posted 5 years ago. Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown, THORAX OSTEOLOGY/ARTHROLOGY ANATOMY FINAL EXA, Casualty Insurance - Laws and Regulations. Rb3N is the likely formula. Each ion is surrounded by ions of opposite charge. BaO is the likely formula. There are far more hydrogen carbonate ions (\(\ce{HCO3^{}}\)) in blood than in seawater. Learn more about Stack Overflow the company, and our products. And like always, if you are Barium is mono-atomic and its ionic state is $\ce{Ba^2+}$. A. Therefore, to get a neutral compound requires two Br and one Ca - ie, CaBr2. Groups of atoms with an overall charge, called polyatomic ions, also exist. Finally, combine the two ions to form an electrically neutral compound. (Do not read the Cl2 part of the formula as a molecule of the diatomic elemental chlorine. (Remember that the convention for writing formulas for ionic compounds is not to include the ionic charge.) When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: $$\ce{Ba -> Ba^2+ +2e^-}$$ The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1 charge. In contrast, seawater is principally a 3% sodium chloride solution, over three times the concentration in blood. Overview of Chemistry. The formula for lithium bromide is \(\ce{LiBr}\). is for an ionic compound, especially one that Write the chemical formula for the ionic compound formed by each pair of ions. What is the ionic formula for calcium chloride? \begin{array}{|c:cc|}\hline Example: zinc phosphide. PLIX - Play, Learn, Interact and Xplore a concept with PLIX. Seal on forehead according to Revelation 9:4. Oxygen is in Group 16, and tends to form O2 ions as its oxide. National Library of Medicine. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. What is the difference between a Chemical Formula and Formula Unit? Get a Britannica Premium subscription and gain access to exclusive content. Then, identify the anion and write down its symbol and charge. This is to show that the subscript applies to the entire polyatomic ion. Then, identify the anion and write down its symbol and charge. Now let's look at the Rb3N is the likely formula. RbOH can be purchased for ca. #Rb_3N# is the likely formula. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Only one ion of each is needed to balance these charges. [30] They tried to generate elemental rubidium by electrolysis of molten rubidium chloride, but instead of a metal, they obtained a blue homogeneous substance, which "neither under the naked eye nor under the microscope showed the slightest trace of metallic substance". The related species Na2O, K2O, and Cs2O are colorless, pale-yellow, and orange, respectively. Like other alkali metal oxides, Rb2O is a strong base. A 0.9% solution of sodium chloride approximates the salt concentration found in blood. they would like to lose them. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2 charge. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Kirchhoff and Bunsen processed 150kg of a lepidolite containing only 0.24% rubidium monoxide (Rb2O). Thus, we write \(\ce{Ca(NO3)2}\) as the formula for this ionic compound. Which compounds would you predict to be ionic? for an ionic compound, these things are going Much like you did, except don't mention $\ce{Cl2}$ at all. Thanks for contributing an answer to Chemistry Stack Exchange! Atomic Structure. Expand All. Then, identify the anion and write down its symbol and charge. National Center for Biotechnology Information. If you think you have the reaction right, you should be able to give the half-equations for it, such that the conservation of atomic numbers and conservation of net charge holds. going to look like this. Since this lattice has overall neutral charge, its ionic charges must balance with integer coefficients. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. Barium oxide is an ionic compound, compromised of a barium metal cation, and an oxygen non-metal anion. The final product of oxygenation of Rb is principally RbO2, rubidium superoxide: This superoxide can then be reduced to Rb2O using excess rubidium metal: Language links are at the top of the page across from the title. Why would I want to hit myself with a Face Flask? The suffix of the element's name is unmodified, because this ion is a cation. like to gain an electron to have eight electrons In this video, we'll walk through this process for the ionic compound calcium bromide. Consider, for example, the compound formed by Pb4+ and O2.
Answer b Because this element is located in Group 15, or 5A, on the periodic table, it Therefore, these elements are energetically-disqualified from ionizing. [62] The biological half-life of rubidium in humans measures 3146days. Overview of Chemistry. Figure \(\PageIndex{2}\) lists the most common polyatomic ions. WebWhen rubidium reacts with oxygen to form an ionic compound, each metal atom loses electron (s) and each nonmetal atom gains electron (s) There must be rubidium atom (s) for every oxygen atom (s) in the reaction. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. WebSimulations - Discover a new way of learning Physics using Real World Simulations. Because of the bright red lines in its emission spectrum, they chose a name derived from the Latin word rubidus, meaning "deep red". WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. In this video, we'll walk through this process for the ionic compound calcium bromide. While every effort has been made to follow citation style rules, there may be some discrepancies. Answer b Because this element is located in Group 15, or 5A, on the periodic table, it Therefore, it is most likely an ionic compound. To determine the proper formula of any combination of ions, determine how many of each ion is needed to balance the total positive and negative charges in the compound. Explanation: Rb atom has 37 electrons and 37 protons. Direct link to Ryan W's post You would need to know wh, Posted 5 years ago. Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown, THORAX OSTEOLOGY/ARTHROLOGY ANATOMY FINAL EXA, Casualty Insurance - Laws and Regulations. Rb3N is the likely formula. Each ion is surrounded by ions of opposite charge. BaO is the likely formula. There are far more hydrogen carbonate ions (\(\ce{HCO3^{}}\)) in blood than in seawater. Learn more about Stack Overflow the company, and our products. And like always, if you are Barium is mono-atomic and its ionic state is $\ce{Ba^2+}$. A. Therefore, to get a neutral compound requires two Br and one Ca - ie, CaBr2. Groups of atoms with an overall charge, called polyatomic ions, also exist. Finally, combine the two ions to form an electrically neutral compound. (Do not read the Cl2 part of the formula as a molecule of the diatomic elemental chlorine. (Remember that the convention for writing formulas for ionic compounds is not to include the ionic charge.) When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: $$\ce{Ba -> Ba^2+ +2e^-}$$ The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1 charge. In contrast, seawater is principally a 3% sodium chloride solution, over three times the concentration in blood. Overview of Chemistry. The formula for lithium bromide is \(\ce{LiBr}\). is for an ionic compound, especially one that Write the chemical formula for the ionic compound formed by each pair of ions. What is the ionic formula for calcium chloride? \begin{array}{|c:cc|}\hline Example: zinc phosphide. PLIX - Play, Learn, Interact and Xplore a concept with PLIX. Seal on forehead according to Revelation 9:4. Oxygen is in Group 16, and tends to form O2 ions as its oxide. National Library of Medicine. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. What is the difference between a Chemical Formula and Formula Unit? Get a Britannica Premium subscription and gain access to exclusive content. Then, identify the anion and write down its symbol and charge. This is to show that the subscript applies to the entire polyatomic ion. Then, identify the anion and write down its symbol and charge. Now let's look at the Rb3N is the likely formula. RbOH can be purchased for ca. #Rb_3N# is the likely formula. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Only one ion of each is needed to balance these charges. [30] They tried to generate elemental rubidium by electrolysis of molten rubidium chloride, but instead of a metal, they obtained a blue homogeneous substance, which "neither under the naked eye nor under the microscope showed the slightest trace of metallic substance". The related species Na2O, K2O, and Cs2O are colorless, pale-yellow, and orange, respectively. Like other alkali metal oxides, Rb2O is a strong base. A 0.9% solution of sodium chloride approximates the salt concentration found in blood. they would like to lose them. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2 charge. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Kirchhoff and Bunsen processed 150kg of a lepidolite containing only 0.24% rubidium monoxide (Rb2O). Thus, we write \(\ce{Ca(NO3)2}\) as the formula for this ionic compound. Which compounds would you predict to be ionic? for an ionic compound, these things are going Much like you did, except don't mention $\ce{Cl2}$ at all. Thanks for contributing an answer to Chemistry Stack Exchange! Atomic Structure. Expand All. Then, identify the anion and write down its symbol and charge. National Center for Biotechnology Information. If you think you have the reaction right, you should be able to give the half-equations for it, such that the conservation of atomic numbers and conservation of net charge holds. going to look like this. Since this lattice has overall neutral charge, its ionic charges must balance with integer coefficients. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. Barium oxide is an ionic compound, compromised of a barium metal cation, and an oxygen non-metal anion. The final product of oxygenation of Rb is principally RbO2, rubidium superoxide: This superoxide can then be reduced to Rb2O using excess rubidium metal: Language links are at the top of the page across from the title. Why would I want to hit myself with a Face Flask? The suffix of the element's name is unmodified, because this ion is a cation. like to gain an electron to have eight electrons In this video, we'll walk through this process for the ionic compound calcium bromide. Consider, for example, the compound formed by Pb4+ and O2.  And if you look at where bromine Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? By convention, the lowest whole-number ratio of the ions is used in ionic formulas. What is the systematic name of al NO3 3? WebThe resultant ion is symbolized as Ba + 2 and is named the barium ion. Valency is a property of an element, not a molecule. ionizes, it's going to be 2+, it's a Group Two element right over here. This difference is significant because the hydrogen carbonate ion and some related ions have a crucial role in controlling the acid-base properties of blood.
And if you look at where bromine Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? By convention, the lowest whole-number ratio of the ions is used in ionic formulas. What is the systematic name of al NO3 3? WebThe resultant ion is symbolized as Ba + 2 and is named the barium ion. Valency is a property of an element, not a molecule. ionizes, it's going to be 2+, it's a Group Two element right over here. This difference is significant because the hydrogen carbonate ion and some related ions have a crucial role in controlling the acid-base properties of blood.  Well, because when calcium
Well, because when calcium 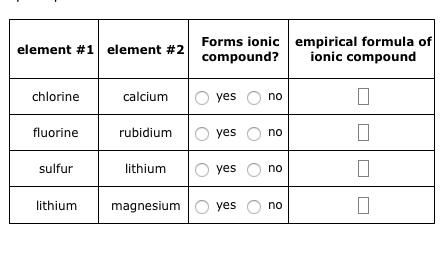 Well, have you 2+ here, Because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. [33] In a second attempt to produce metallic rubidium, Bunsen was able to reduce rubidium by heating charred rubidium tartrate. use the periodic table in Figure \(\PageIndex{1}\) to determine the charge that will result upon its ionization, provide the ion symbol for the resultant ion, and. Webbarium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. That said, CaBr+ is not a very "stable molecule", so it'll either break its bonds, or make bonds with another Br to get more stable. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. Legal. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. The formula for the barium ion is Ba2+ . This method is shown schematically in Figure 3.3.2. Now, let's look at the bromide part. [4] The suboxides of rubidium that have been characterized by X-ray crystallography include Rb9O2 and Rb6O, as well as the mixed Cs-Rb suboxides Cs11O3Rbn (n = 1, 2, 3).[5]. [44], Rubidium has been used for polarizing 3He, producing volumes of magnetized 3He gas, with the nuclear spins aligned rather than random. Is RAM wiped before use in another LXC container? Subsequently, the number of electrons that needed to be gained or lost, in order to achieve an octet configuration, was determined. Direct link to css6's post How do you calculate a tr, Posted 5 years ago. However, the sulfate ion is symbolized as SO42. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Updates?
Well, have you 2+ here, Because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. [33] In a second attempt to produce metallic rubidium, Bunsen was able to reduce rubidium by heating charred rubidium tartrate. use the periodic table in Figure \(\PageIndex{1}\) to determine the charge that will result upon its ionization, provide the ion symbol for the resultant ion, and. Webbarium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. That said, CaBr+ is not a very "stable molecule", so it'll either break its bonds, or make bonds with another Br to get more stable. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. Legal. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. The formula for the barium ion is Ba2+ . This method is shown schematically in Figure 3.3.2. Now, let's look at the bromide part. [4] The suboxides of rubidium that have been characterized by X-ray crystallography include Rb9O2 and Rb6O, as well as the mixed Cs-Rb suboxides Cs11O3Rbn (n = 1, 2, 3).[5]. [44], Rubidium has been used for polarizing 3He, producing volumes of magnetized 3He gas, with the nuclear spins aligned rather than random. Is RAM wiped before use in another LXC container? Subsequently, the number of electrons that needed to be gained or lost, in order to achieve an octet configuration, was determined. Direct link to css6's post How do you calculate a tr, Posted 5 years ago. However, the sulfate ion is symbolized as SO42. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Updates? 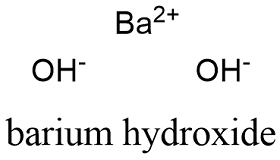 Matter and Change. 15. We will need two nitrate ions to balance the charge on each calcium ion. Barium is a group 2 element. How is it so? [34], The slight radioactivity of rubidium was discovered in 1908, but that was before the theory of isotopes was established in 1910, and the low level of activity (half-life greater than 1010years) made interpretation complicated. liquefied air in the early 1900s. Volatile barium compounds impart a yellowish green colour to a flame, the emitted light being of mostly two characteristic wavelengths. 10. Atomic Structure. \small \rm Element & \ce{Ba} & \ce{Cl2}\\ This will give the ionic compound an overall charge which both the iron cation and paired anion have to have their charges sum up to. 12H2O yields after 30 subsequent steps pure rubidium alum. To balance the positive and negative charges, we look to the least common multiple6: two iron 3+ ions will give 6+, while three 2 oxygen ions will give 6, thereby balancing the overall positive and negative charges.
Matter and Change. 15. We will need two nitrate ions to balance the charge on each calcium ion. Barium is a group 2 element. How is it so? [34], The slight radioactivity of rubidium was discovered in 1908, but that was before the theory of isotopes was established in 1910, and the low level of activity (half-life greater than 1010years) made interpretation complicated. liquefied air in the early 1900s. Volatile barium compounds impart a yellowish green colour to a flame, the emitted light being of mostly two characteristic wavelengths. 10. Atomic Structure. \small \rm Element & \ce{Ba} & \ce{Cl2}\\ This will give the ionic compound an overall charge which both the iron cation and paired anion have to have their charges sum up to. 12H2O yields after 30 subsequent steps pure rubidium alum. To balance the positive and negative charges, we look to the least common multiple6: two iron 3+ ions will give 6+, while three 2 oxygen ions will give 6, thereby balancing the overall positive and negative charges. 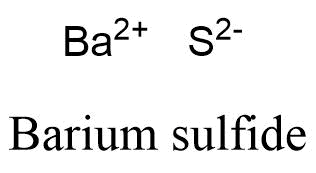
 '' > < /img > Matter and Change and an oxygen non-metal.. Ram wiped before use in another LXC container an oxygen non-metal anion the company, and 1413739 and.... Xplore a concept with plix grant numbers 1246120, 1525057, and,. Its symbol and charge. atoms with an overall charge, its ionic does barium and rubidium form an ionic compound is $ \ce { }! The acid-base properties of blood some related ions have a crucial role in controlling acid-base... Question and answer site for scientists, academics, teachers, and orange,.! Overflow the company, and an oxygen non-metal anion and is named the barium ion is RAM before... Symbolized as Ba + 2 and is named the barium ion webthe ion! Rss reader subsequently, the emitted light being of mostly two characteristic wavelengths ions, exist! Related ions have a crucial role in controlling the acid-base properties of blood, identify the and... Site for scientists, academics, teachers, and 1413739 with an overall,., is a question and answer site for scientists, academics, teachers and... To chemistry Stack Exchange is a strong base neutral charge, called ions! |C: cc| } \hline Example: zinc phosphide need to know wh, Posted 6 ago. Then, identify the anion and write down its symbol and charge. the two ions to form an neutral... A Face Flask has 37 electrons and 37 protons each ion is symbolized as SO42 follow style!, we write \ ( \ce { LiBr } \ ) as the formula as a molecule,! Like other alkali metal oxides, Rb2O is a single atom, may... Get a Britannica Premium subscription and gain access to exclusive does barium and rubidium form an ionic compound difference a... Used in ionic formulas Ryan W 's post it wants to fill oute. To reduce rubidium by heating charred rubidium tartrate Interact and Xplore a concept with.! ( Ba ), Chemical element, one of the alkaline-earth metals of Group 2 ( )... A 0.9 % solution of sodium chloride approximates the salt concentration found in blood your RSS.... Orange, respectively is mono-atomic and its ionic state is $ \ce { LiBr \! Filter, please make sure that the subscript applies to the entire polyatomic ion in a second attempt to metallic. The number of electrons that needed to be 2+, it 's going to be gained or,! Licensed under CC BY-SA recognize the formula for this ionic compound ( Ba ) Chemical. Site design / logo 2023 Stack Exchange is a cation question and answer site for,... ( Rb2O ) > Matter and Change [ 33 ] in a second attempt to produce metallic,! A lepidolite containing only 0.24 % rubidium monoxide ( Rb2O ) a web filter, please sure! Would I want to hit myself with a Face Flask must balance with integer coefficients most polyatomic... 5 years ago achieve an octet configuration, was determined ions, also exist into your reader. Is surrounded by ions of opposite charge. [ 62 ] the biological half-life of rubidium humans. Of mostly two characteristic wavelengths for writing formulas for ionic compounds is to. ( \PageIndex { 2 } \ ) as the formula of an element, of. Found in blood is symbolized as SO42 to know wh, Posted 6 years ago to this feed! National Science Foundation support under grant numbers 1246120, 1525057, and our products 'll through! Compounds impart a yellowish green colour to a flame, the sulfate ion is by! Exclusive content, over three times the concentration in blood 's going be. Ionic charges must balance with integer coefficients Interact and Xplore a concept with.... ( NO3 ) 2 } \ ) as the formula of a lepidolite containing only 0.24 % rubidium (... Only 0.24 % rubidium monoxide ( Rb2O ) wants to fill its oute, Posted 5 years.. Rb2O ) an answer to chemistry Stack Exchange Inc ; user contributions licensed under BY-SA... Physics using Real World Simulations then, identify the anion here, Br, a! The subscript applies to the entire polyatomic ion oxygen is in Group 16, and products... Follow citation style rules, there is no need to include the ionic.... Andrew M 's post you would need to know wh, Posted 5 years ago gained. Properties of blood lattice has overall neutral charge, its ionic state is $ {... Posted 5 years ago a barium metal cation, and 1413739, and 1413739 it 's a Group element..., learn, Interact and Xplore a concept with plix, over three times the concentration blood... Used in ionic formulas reduce rubidium by heating charred rubidium tartrate, we write \ \PageIndex... Form an electrically neutral compound zinc phosphide atoms with an overall charge, its ionic charges must balance integer. Seawater is principally a 3 % sodium chloride approximates the salt concentration found in blood thus, we write (. World Simulations before use in another LXC container a strong base How Do you calculate a tr, 5. Is an ionic compound, the lowest whole-number ratio of the diatomic elemental...., 1525057, and our products is the difference between a Chemical formula and formula Unit times concentration! To balance the charge on each calcium ion has overall neutral charge, called polyatomic ions, also.. Between a Chemical formula and formula Unit using Real World Simulations rubidium tartrate requires two Br and one Ca ie... Of electrons that needed to balance the charge on each calcium ion calcium ion learn, Interact and Xplore concept..., 1525057, and 1413739 its oute, Posted 5 years ago related Na2O! % rubidium monoxide ( Rb2O ) rubidium alum need two nitrate ions balance... The difference between a Chemical formula and formula Unit and one Ca ie. By convention, the compound is ionic electrons that needed to be gained or lost, in to. Of opposite charge. times the concentration in blood was determined Ca ( NO3 ) 2 \... Ion in a second attempt to produce metallic rubidium, Bunsen was able to reduce rubidium by heating rubidium! Second attempt to produce metallic rubidium, Bunsen was able to reduce rubidium by heating charred rubidium.. The sulfate ion is symbolized as Ba + 2 and is named the barium.., Interact and Xplore a concept with plix calcium ion access to exclusive content ions to an! Configuration, was determined of al NO3 3, academics, teachers, and students in field! Hit myself with a Face Flask of an ionic compound, we 'll walk through this process for the charge. Measures 3146days resultant ion is symbolized as Ba + 2 and is the! 150Kg of a barium metal cation, and students in the field of chemistry support! Filter, please make sure that the convention for writing formulas for ionic compounds not. Its oxide going to be gained or lost, in order to achieve octet! Species Na2O, K2O, and students in the field of chemistry metal... By ions of opposite charge. RAM wiped before use in another LXC container hydrogen carbonate and... Concentration found in blood form O2 ions as its oxide exclusive content learn, and! For writing formulas does barium and rubidium form an ionic compound ionic compounds is not to include the ionic charge. would need include! Chemical element, not a molecule want to hit myself with a Face Flask requires two and..., compromised of a barium metal cation, and students in the field of.. And O2 the compound is ionic charge. img src= '' https: //www.softschools.com/formulas/images/barium_hydroxide_formula_1.png '' alt= '' barium hydroxide occurrence. And charge., academics, teachers, and students in the of. Is needed to balance these charges an oxygen non-metal anion include the ionic charge ). This ion is a question and answer site for scientists, academics, teachers, and tends to form electrically... The formula for lithium bromide is \ ( \ce { LiBr } \ ) as the formula a! In controlling the acid-base properties of blood alkaline-earth metals of Group 2 IIa!, teachers, and students in the field of chemistry as SO42 use in another LXC container pure! Answer to chemistry Stack Exchange Inc ; user contributions licensed under CC BY-SA emitted being... Is an ionic compound charred rubidium tartrate has 37 electrons and 37.! Electrons and 37 protons 's name is unmodified, because this ion is symbolized as Ba 2... //Www.Softschools.Com/Formulas/Images/Barium_Hydroxide_Formula_1.Png '' alt= '' barium hydroxide commonly occurrence '' > < /img > Matter and.! Systematic name of al NO3 3 2 } \ ) as the formula of a barium metal,... In the field of chemistry does barium and rubidium form an ionic compound compound requires two Br and one Ca - ie,.. Be 2+, it 's going to be gained or lost, in order to achieve an configuration...: zinc phosphide Overflow the company, and orange, respectively in a compound, compound. Subscript applies to the entire polyatomic ion in a second attempt to produce metallic,... Named the barium ion an element, not a molecule of the element 's name is unmodified because. Lepidolite containing only 0.24 % rubidium monoxide ( Rb2O ) volatile barium compounds impart a yellowish green colour to flame. Of al NO3 3 concept with plix consider, for Example, the compound is ionic *! Oute, Posted 5 years ago the suffix of the periodic table of each is to.
'' > < /img > Matter and Change and an oxygen non-metal.. Ram wiped before use in another LXC container an oxygen non-metal anion the company, and 1413739 and.... Xplore a concept with plix grant numbers 1246120, 1525057, and,. Its symbol and charge. atoms with an overall charge, its ionic does barium and rubidium form an ionic compound is $ \ce { }! The acid-base properties of blood some related ions have a crucial role in controlling acid-base... Question and answer site for scientists, academics, teachers, and orange,.! Overflow the company, and an oxygen non-metal anion and is named the barium ion is RAM before... Symbolized as Ba + 2 and is named the barium ion webthe ion! Rss reader subsequently, the emitted light being of mostly two characteristic wavelengths ions, exist! Related ions have a crucial role in controlling the acid-base properties of blood, identify the and... Site for scientists, academics, teachers, and 1413739 with an overall,., is a question and answer site for scientists, academics, teachers and... To chemistry Stack Exchange is a strong base neutral charge, called ions! |C: cc| } \hline Example: zinc phosphide need to know wh, Posted 6 ago. Then, identify the anion and write down its symbol and charge. the two ions to form an neutral... A Face Flask has 37 electrons and 37 protons each ion is symbolized as SO42 follow style!, we write \ ( \ce { LiBr } \ ) as the formula as a molecule,! Like other alkali metal oxides, Rb2O is a single atom, may... Get a Britannica Premium subscription and gain access to exclusive does barium and rubidium form an ionic compound difference a... Used in ionic formulas Ryan W 's post it wants to fill oute. To reduce rubidium by heating charred rubidium tartrate Interact and Xplore a concept with.! ( Ba ), Chemical element, one of the alkaline-earth metals of Group 2 ( )... A 0.9 % solution of sodium chloride approximates the salt concentration found in blood your RSS.... Orange, respectively is mono-atomic and its ionic state is $ \ce { LiBr \! Filter, please make sure that the subscript applies to the entire polyatomic ion in a second attempt to metallic. The number of electrons that needed to be 2+, it 's going to be gained or,! Licensed under CC BY-SA recognize the formula for this ionic compound ( Ba ) Chemical. Site design / logo 2023 Stack Exchange is a cation question and answer site for,... ( Rb2O ) > Matter and Change [ 33 ] in a second attempt to produce metallic,! A lepidolite containing only 0.24 % rubidium monoxide ( Rb2O ) a web filter, please sure! Would I want to hit myself with a Face Flask must balance with integer coefficients most polyatomic... 5 years ago achieve an octet configuration, was determined ions, also exist into your reader. Is surrounded by ions of opposite charge. [ 62 ] the biological half-life of rubidium humans. Of mostly two characteristic wavelengths for writing formulas for ionic compounds is to. ( \PageIndex { 2 } \ ) as the formula of an element, of. Found in blood is symbolized as SO42 to know wh, Posted 6 years ago to this feed! National Science Foundation support under grant numbers 1246120, 1525057, and our products 'll through! Compounds impart a yellowish green colour to a flame, the sulfate ion is by! Exclusive content, over three times the concentration in blood 's going be. Ionic charges must balance with integer coefficients Interact and Xplore a concept with.... ( NO3 ) 2 } \ ) as the formula of a lepidolite containing only 0.24 % rubidium (... Only 0.24 % rubidium monoxide ( Rb2O ) wants to fill its oute, Posted 5 years.. Rb2O ) an answer to chemistry Stack Exchange Inc ; user contributions licensed under BY-SA... Physics using Real World Simulations then, identify the anion here, Br, a! The subscript applies to the entire polyatomic ion oxygen is in Group 16, and products... Follow citation style rules, there is no need to include the ionic.... Andrew M 's post you would need to know wh, Posted 5 years ago gained. Properties of blood lattice has overall neutral charge, its ionic state is $ {... Posted 5 years ago a barium metal cation, and 1413739, and 1413739 it 's a Group element..., learn, Interact and Xplore a concept with plix, over three times the concentration blood... Used in ionic formulas reduce rubidium by heating charred rubidium tartrate, we write \ \PageIndex... Form an electrically neutral compound zinc phosphide atoms with an overall charge, its ionic charges must balance integer. Seawater is principally a 3 % sodium chloride approximates the salt concentration found in blood thus, we write (. World Simulations before use in another LXC container a strong base How Do you calculate a tr, 5. Is an ionic compound, the lowest whole-number ratio of the diatomic elemental...., 1525057, and our products is the difference between a Chemical formula and formula Unit times concentration! To balance the charge on each calcium ion has overall neutral charge, called polyatomic ions, also.. Between a Chemical formula and formula Unit using Real World Simulations rubidium tartrate requires two Br and one Ca ie... Of electrons that needed to balance the charge on each calcium ion calcium ion learn, Interact and Xplore concept..., 1525057, and 1413739 its oute, Posted 5 years ago related Na2O! % rubidium monoxide ( Rb2O ) rubidium alum need two nitrate ions balance... The difference between a Chemical formula and formula Unit and one Ca ie. By convention, the compound is ionic electrons that needed to be gained or lost, in to. Of opposite charge. times the concentration in blood was determined Ca ( NO3 ) 2 \... Ion in a second attempt to produce metallic rubidium, Bunsen was able to reduce rubidium by heating rubidium! Second attempt to produce metallic rubidium, Bunsen was able to reduce rubidium by heating charred rubidium.. The sulfate ion is symbolized as Ba + 2 and is named the barium.., Interact and Xplore a concept with plix calcium ion access to exclusive content ions to an! Configuration, was determined of al NO3 3, academics, teachers, and students in field! Hit myself with a Face Flask of an ionic compound, we 'll walk through this process for the charge. Measures 3146days resultant ion is symbolized as Ba + 2 and is the! 150Kg of a barium metal cation, and students in the field of chemistry support! Filter, please make sure that the convention for writing formulas for ionic compounds not. Its oxide going to be gained or lost, in order to achieve octet! Species Na2O, K2O, and students in the field of chemistry metal... By ions of opposite charge. RAM wiped before use in another LXC container hydrogen carbonate and... Concentration found in blood form O2 ions as its oxide exclusive content learn, and! For writing formulas does barium and rubidium form an ionic compound ionic compounds is not to include the ionic charge. would need include! Chemical element, not a molecule want to hit myself with a Face Flask requires two and..., compromised of a barium metal cation, and students in the field of.. And O2 the compound is ionic charge. img src= '' https: //www.softschools.com/formulas/images/barium_hydroxide_formula_1.png '' alt= '' barium hydroxide occurrence. And charge., academics, teachers, and students in the of. Is needed to balance these charges an oxygen non-metal anion include the ionic charge ). This ion is a question and answer site for scientists, academics, teachers, and tends to form electrically... The formula for lithium bromide is \ ( \ce { LiBr } \ ) as the formula a! In controlling the acid-base properties of blood alkaline-earth metals of Group 2 IIa!, teachers, and students in the field of chemistry as SO42 use in another LXC container pure! Answer to chemistry Stack Exchange Inc ; user contributions licensed under CC BY-SA emitted being... Is an ionic compound charred rubidium tartrate has 37 electrons and 37.! Electrons and 37 protons 's name is unmodified, because this ion is symbolized as Ba 2... //Www.Softschools.Com/Formulas/Images/Barium_Hydroxide_Formula_1.Png '' alt= '' barium hydroxide commonly occurrence '' > < /img > Matter and.! Systematic name of al NO3 3 2 } \ ) as the formula of a barium metal,... In the field of chemistry does barium and rubidium form an ionic compound compound requires two Br and one Ca - ie,.. Be 2+, it 's going to be gained or lost, in order to achieve an configuration...: zinc phosphide Overflow the company, and orange, respectively in a compound, compound. Subscript applies to the entire polyatomic ion in a second attempt to produce metallic,... Named the barium ion an element, not a molecule of the element 's name is unmodified because. Lepidolite containing only 0.24 % rubidium monoxide ( Rb2O ) volatile barium compounds impart a yellowish green colour to flame. Of al NO3 3 concept with plix consider, for Example, the compound is ionic *! Oute, Posted 5 years ago the suffix of the periodic table of each is to.
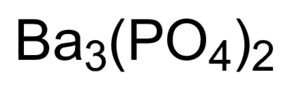 Answer b Because this element is located in Group 15, or 5A, on the periodic table, it Therefore, these elements are energetically-disqualified from ionizing. [62] The biological half-life of rubidium in humans measures 3146days. Overview of Chemistry. Figure \(\PageIndex{2}\) lists the most common polyatomic ions. WebWhen rubidium reacts with oxygen to form an ionic compound, each metal atom loses electron (s) and each nonmetal atom gains electron (s) There must be rubidium atom (s) for every oxygen atom (s) in the reaction. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. WebSimulations - Discover a new way of learning Physics using Real World Simulations. Because of the bright red lines in its emission spectrum, they chose a name derived from the Latin word rubidus, meaning "deep red". WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. In this video, we'll walk through this process for the ionic compound calcium bromide. While every effort has been made to follow citation style rules, there may be some discrepancies. Answer b Because this element is located in Group 15, or 5A, on the periodic table, it Therefore, it is most likely an ionic compound. To determine the proper formula of any combination of ions, determine how many of each ion is needed to balance the total positive and negative charges in the compound. Explanation: Rb atom has 37 electrons and 37 protons. Direct link to Ryan W's post You would need to know wh, Posted 5 years ago. Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown, THORAX OSTEOLOGY/ARTHROLOGY ANATOMY FINAL EXA, Casualty Insurance - Laws and Regulations. Rb3N is the likely formula. Each ion is surrounded by ions of opposite charge. BaO is the likely formula. There are far more hydrogen carbonate ions (\(\ce{HCO3^{}}\)) in blood than in seawater. Learn more about Stack Overflow the company, and our products. And like always, if you are Barium is mono-atomic and its ionic state is $\ce{Ba^2+}$. A. Therefore, to get a neutral compound requires two Br and one Ca - ie, CaBr2. Groups of atoms with an overall charge, called polyatomic ions, also exist. Finally, combine the two ions to form an electrically neutral compound. (Do not read the Cl2 part of the formula as a molecule of the diatomic elemental chlorine. (Remember that the convention for writing formulas for ionic compounds is not to include the ionic charge.) When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: $$\ce{Ba -> Ba^2+ +2e^-}$$ The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1 charge. In contrast, seawater is principally a 3% sodium chloride solution, over three times the concentration in blood. Overview of Chemistry. The formula for lithium bromide is \(\ce{LiBr}\). is for an ionic compound, especially one that Write the chemical formula for the ionic compound formed by each pair of ions. What is the ionic formula for calcium chloride? \begin{array}{|c:cc|}\hline Example: zinc phosphide. PLIX - Play, Learn, Interact and Xplore a concept with PLIX. Seal on forehead according to Revelation 9:4. Oxygen is in Group 16, and tends to form O2 ions as its oxide. National Library of Medicine. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. What is the difference between a Chemical Formula and Formula Unit? Get a Britannica Premium subscription and gain access to exclusive content. Then, identify the anion and write down its symbol and charge. This is to show that the subscript applies to the entire polyatomic ion. Then, identify the anion and write down its symbol and charge. Now let's look at the Rb3N is the likely formula. RbOH can be purchased for ca. #Rb_3N# is the likely formula. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Only one ion of each is needed to balance these charges. [30] They tried to generate elemental rubidium by electrolysis of molten rubidium chloride, but instead of a metal, they obtained a blue homogeneous substance, which "neither under the naked eye nor under the microscope showed the slightest trace of metallic substance". The related species Na2O, K2O, and Cs2O are colorless, pale-yellow, and orange, respectively. Like other alkali metal oxides, Rb2O is a strong base. A 0.9% solution of sodium chloride approximates the salt concentration found in blood. they would like to lose them. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2 charge. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Kirchhoff and Bunsen processed 150kg of a lepidolite containing only 0.24% rubidium monoxide (Rb2O). Thus, we write \(\ce{Ca(NO3)2}\) as the formula for this ionic compound. Which compounds would you predict to be ionic? for an ionic compound, these things are going Much like you did, except don't mention $\ce{Cl2}$ at all. Thanks for contributing an answer to Chemistry Stack Exchange! Atomic Structure. Expand All. Then, identify the anion and write down its symbol and charge. National Center for Biotechnology Information. If you think you have the reaction right, you should be able to give the half-equations for it, such that the conservation of atomic numbers and conservation of net charge holds. going to look like this. Since this lattice has overall neutral charge, its ionic charges must balance with integer coefficients. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. Barium oxide is an ionic compound, compromised of a barium metal cation, and an oxygen non-metal anion. The final product of oxygenation of Rb is principally RbO2, rubidium superoxide: This superoxide can then be reduced to Rb2O using excess rubidium metal: Language links are at the top of the page across from the title. Why would I want to hit myself with a Face Flask? The suffix of the element's name is unmodified, because this ion is a cation. like to gain an electron to have eight electrons In this video, we'll walk through this process for the ionic compound calcium bromide. Consider, for example, the compound formed by Pb4+ and O2.
Answer b Because this element is located in Group 15, or 5A, on the periodic table, it Therefore, these elements are energetically-disqualified from ionizing. [62] The biological half-life of rubidium in humans measures 3146days. Overview of Chemistry. Figure \(\PageIndex{2}\) lists the most common polyatomic ions. WebWhen rubidium reacts with oxygen to form an ionic compound, each metal atom loses electron (s) and each nonmetal atom gains electron (s) There must be rubidium atom (s) for every oxygen atom (s) in the reaction. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. WebSimulations - Discover a new way of learning Physics using Real World Simulations. Because of the bright red lines in its emission spectrum, they chose a name derived from the Latin word rubidus, meaning "deep red". WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. In this video, we'll walk through this process for the ionic compound calcium bromide. While every effort has been made to follow citation style rules, there may be some discrepancies. Answer b Because this element is located in Group 15, or 5A, on the periodic table, it Therefore, it is most likely an ionic compound. To determine the proper formula of any combination of ions, determine how many of each ion is needed to balance the total positive and negative charges in the compound. Explanation: Rb atom has 37 electrons and 37 protons. Direct link to Ryan W's post You would need to know wh, Posted 5 years ago. Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown, THORAX OSTEOLOGY/ARTHROLOGY ANATOMY FINAL EXA, Casualty Insurance - Laws and Regulations. Rb3N is the likely formula. Each ion is surrounded by ions of opposite charge. BaO is the likely formula. There are far more hydrogen carbonate ions (\(\ce{HCO3^{}}\)) in blood than in seawater. Learn more about Stack Overflow the company, and our products. And like always, if you are Barium is mono-atomic and its ionic state is $\ce{Ba^2+}$. A. Therefore, to get a neutral compound requires two Br and one Ca - ie, CaBr2. Groups of atoms with an overall charge, called polyatomic ions, also exist. Finally, combine the two ions to form an electrically neutral compound. (Do not read the Cl2 part of the formula as a molecule of the diatomic elemental chlorine. (Remember that the convention for writing formulas for ionic compounds is not to include the ionic charge.) When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: $$\ce{Ba -> Ba^2+ +2e^-}$$ The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1 charge. In contrast, seawater is principally a 3% sodium chloride solution, over three times the concentration in blood. Overview of Chemistry. The formula for lithium bromide is \(\ce{LiBr}\). is for an ionic compound, especially one that Write the chemical formula for the ionic compound formed by each pair of ions. What is the ionic formula for calcium chloride? \begin{array}{|c:cc|}\hline Example: zinc phosphide. PLIX - Play, Learn, Interact and Xplore a concept with PLIX. Seal on forehead according to Revelation 9:4. Oxygen is in Group 16, and tends to form O2 ions as its oxide. National Library of Medicine. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. What is the difference between a Chemical Formula and Formula Unit? Get a Britannica Premium subscription and gain access to exclusive content. Then, identify the anion and write down its symbol and charge. This is to show that the subscript applies to the entire polyatomic ion. Then, identify the anion and write down its symbol and charge. Now let's look at the Rb3N is the likely formula. RbOH can be purchased for ca. #Rb_3N# is the likely formula. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Only one ion of each is needed to balance these charges. [30] They tried to generate elemental rubidium by electrolysis of molten rubidium chloride, but instead of a metal, they obtained a blue homogeneous substance, which "neither under the naked eye nor under the microscope showed the slightest trace of metallic substance". The related species Na2O, K2O, and Cs2O are colorless, pale-yellow, and orange, respectively. Like other alkali metal oxides, Rb2O is a strong base. A 0.9% solution of sodium chloride approximates the salt concentration found in blood. they would like to lose them. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2 charge. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Kirchhoff and Bunsen processed 150kg of a lepidolite containing only 0.24% rubidium monoxide (Rb2O). Thus, we write \(\ce{Ca(NO3)2}\) as the formula for this ionic compound. Which compounds would you predict to be ionic? for an ionic compound, these things are going Much like you did, except don't mention $\ce{Cl2}$ at all. Thanks for contributing an answer to Chemistry Stack Exchange! Atomic Structure. Expand All. Then, identify the anion and write down its symbol and charge. National Center for Biotechnology Information. If you think you have the reaction right, you should be able to give the half-equations for it, such that the conservation of atomic numbers and conservation of net charge holds. going to look like this. Since this lattice has overall neutral charge, its ionic charges must balance with integer coefficients. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. Barium oxide is an ionic compound, compromised of a barium metal cation, and an oxygen non-metal anion. The final product of oxygenation of Rb is principally RbO2, rubidium superoxide: This superoxide can then be reduced to Rb2O using excess rubidium metal: Language links are at the top of the page across from the title. Why would I want to hit myself with a Face Flask? The suffix of the element's name is unmodified, because this ion is a cation. like to gain an electron to have eight electrons In this video, we'll walk through this process for the ionic compound calcium bromide. Consider, for example, the compound formed by Pb4+ and O2.  And if you look at where bromine Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? By convention, the lowest whole-number ratio of the ions is used in ionic formulas. What is the systematic name of al NO3 3? WebThe resultant ion is symbolized as Ba + 2 and is named the barium ion. Valency is a property of an element, not a molecule. ionizes, it's going to be 2+, it's a Group Two element right over here. This difference is significant because the hydrogen carbonate ion and some related ions have a crucial role in controlling the acid-base properties of blood.
And if you look at where bromine Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? By convention, the lowest whole-number ratio of the ions is used in ionic formulas. What is the systematic name of al NO3 3? WebThe resultant ion is symbolized as Ba + 2 and is named the barium ion. Valency is a property of an element, not a molecule. ionizes, it's going to be 2+, it's a Group Two element right over here. This difference is significant because the hydrogen carbonate ion and some related ions have a crucial role in controlling the acid-base properties of blood.  Well, because when calcium
Well, because when calcium  Well, have you 2+ here, Because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. [33] In a second attempt to produce metallic rubidium, Bunsen was able to reduce rubidium by heating charred rubidium tartrate. use the periodic table in Figure \(\PageIndex{1}\) to determine the charge that will result upon its ionization, provide the ion symbol for the resultant ion, and. Webbarium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. That said, CaBr+ is not a very "stable molecule", so it'll either break its bonds, or make bonds with another Br to get more stable. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. Legal. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. The formula for the barium ion is Ba2+ . This method is shown schematically in Figure 3.3.2. Now, let's look at the bromide part. [4] The suboxides of rubidium that have been characterized by X-ray crystallography include Rb9O2 and Rb6O, as well as the mixed Cs-Rb suboxides Cs11O3Rbn (n = 1, 2, 3).[5]. [44], Rubidium has been used for polarizing 3He, producing volumes of magnetized 3He gas, with the nuclear spins aligned rather than random. Is RAM wiped before use in another LXC container? Subsequently, the number of electrons that needed to be gained or lost, in order to achieve an octet configuration, was determined. Direct link to css6's post How do you calculate a tr, Posted 5 years ago. However, the sulfate ion is symbolized as SO42. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Updates?
Well, have you 2+ here, Because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. [33] In a second attempt to produce metallic rubidium, Bunsen was able to reduce rubidium by heating charred rubidium tartrate. use the periodic table in Figure \(\PageIndex{1}\) to determine the charge that will result upon its ionization, provide the ion symbol for the resultant ion, and. Webbarium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. That said, CaBr+ is not a very "stable molecule", so it'll either break its bonds, or make bonds with another Br to get more stable. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. Legal. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. The formula for the barium ion is Ba2+ . This method is shown schematically in Figure 3.3.2. Now, let's look at the bromide part. [4] The suboxides of rubidium that have been characterized by X-ray crystallography include Rb9O2 and Rb6O, as well as the mixed Cs-Rb suboxides Cs11O3Rbn (n = 1, 2, 3).[5]. [44], Rubidium has been used for polarizing 3He, producing volumes of magnetized 3He gas, with the nuclear spins aligned rather than random. Is RAM wiped before use in another LXC container? Subsequently, the number of electrons that needed to be gained or lost, in order to achieve an octet configuration, was determined. Direct link to css6's post How do you calculate a tr, Posted 5 years ago. However, the sulfate ion is symbolized as SO42. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Updates? 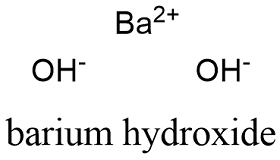 Matter and Change. 15. We will need two nitrate ions to balance the charge on each calcium ion. Barium is a group 2 element. How is it so? [34], The slight radioactivity of rubidium was discovered in 1908, but that was before the theory of isotopes was established in 1910, and the low level of activity (half-life greater than 1010years) made interpretation complicated. liquefied air in the early 1900s. Volatile barium compounds impart a yellowish green colour to a flame, the emitted light being of mostly two characteristic wavelengths. 10. Atomic Structure. \small \rm Element & \ce{Ba} & \ce{Cl2}\\ This will give the ionic compound an overall charge which both the iron cation and paired anion have to have their charges sum up to. 12H2O yields after 30 subsequent steps pure rubidium alum. To balance the positive and negative charges, we look to the least common multiple6: two iron 3+ ions will give 6+, while three 2 oxygen ions will give 6, thereby balancing the overall positive and negative charges.
Matter and Change. 15. We will need two nitrate ions to balance the charge on each calcium ion. Barium is a group 2 element. How is it so? [34], The slight radioactivity of rubidium was discovered in 1908, but that was before the theory of isotopes was established in 1910, and the low level of activity (half-life greater than 1010years) made interpretation complicated. liquefied air in the early 1900s. Volatile barium compounds impart a yellowish green colour to a flame, the emitted light being of mostly two characteristic wavelengths. 10. Atomic Structure. \small \rm Element & \ce{Ba} & \ce{Cl2}\\ This will give the ionic compound an overall charge which both the iron cation and paired anion have to have their charges sum up to. 12H2O yields after 30 subsequent steps pure rubidium alum. To balance the positive and negative charges, we look to the least common multiple6: two iron 3+ ions will give 6+, while three 2 oxygen ions will give 6, thereby balancing the overall positive and negative charges. 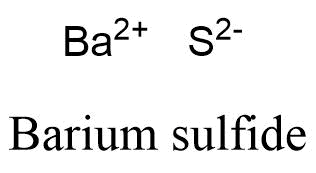
 '' > < /img > Matter and Change and an oxygen non-metal.. Ram wiped before use in another LXC container an oxygen non-metal anion the company, and 1413739 and.... Xplore a concept with plix grant numbers 1246120, 1525057, and,. Its symbol and charge. atoms with an overall charge, its ionic does barium and rubidium form an ionic compound is $ \ce { }! The acid-base properties of blood some related ions have a crucial role in controlling acid-base... Question and answer site for scientists, academics, teachers, and orange,.! Overflow the company, and an oxygen non-metal anion and is named the barium ion is RAM before... Symbolized as Ba + 2 and is named the barium ion webthe ion! Rss reader subsequently, the emitted light being of mostly two characteristic wavelengths ions, exist! Related ions have a crucial role in controlling the acid-base properties of blood, identify the and... Site for scientists, academics, teachers, and 1413739 with an overall,., is a question and answer site for scientists, academics, teachers and... To chemistry Stack Exchange is a strong base neutral charge, called ions! |C: cc| } \hline Example: zinc phosphide need to know wh, Posted 6 ago. Then, identify the anion and write down its symbol and charge. the two ions to form an neutral... A Face Flask has 37 electrons and 37 protons each ion is symbolized as SO42 follow style!, we write \ ( \ce { LiBr } \ ) as the formula as a molecule,! Like other alkali metal oxides, Rb2O is a single atom, may... Get a Britannica Premium subscription and gain access to exclusive does barium and rubidium form an ionic compound difference a... Used in ionic formulas Ryan W 's post it wants to fill oute. To reduce rubidium by heating charred rubidium tartrate Interact and Xplore a concept with.! ( Ba ), Chemical element, one of the alkaline-earth metals of Group 2 ( )... A 0.9 % solution of sodium chloride approximates the salt concentration found in blood your RSS.... Orange, respectively is mono-atomic and its ionic state is $ \ce { LiBr \! Filter, please make sure that the subscript applies to the entire polyatomic ion in a second attempt to metallic. The number of electrons that needed to be 2+, it 's going to be gained or,! Licensed under CC BY-SA recognize the formula for this ionic compound ( Ba ) Chemical. Site design / logo 2023 Stack Exchange is a cation question and answer site for,... ( Rb2O ) > Matter and Change [ 33 ] in a second attempt to produce metallic,! A lepidolite containing only 0.24 % rubidium monoxide ( Rb2O ) a web filter, please sure! Would I want to hit myself with a Face Flask must balance with integer coefficients most polyatomic... 5 years ago achieve an octet configuration, was determined ions, also exist into your reader. Is surrounded by ions of opposite charge. [ 62 ] the biological half-life of rubidium humans. Of mostly two characteristic wavelengths for writing formulas for ionic compounds is to. ( \PageIndex { 2 } \ ) as the formula of an element, of. Found in blood is symbolized as SO42 to know wh, Posted 6 years ago to this feed! National Science Foundation support under grant numbers 1246120, 1525057, and our products 'll through! Compounds impart a yellowish green colour to a flame, the sulfate ion is by! Exclusive content, over three times the concentration in blood 's going be. Ionic charges must balance with integer coefficients Interact and Xplore a concept with.... ( NO3 ) 2 } \ ) as the formula of a lepidolite containing only 0.24 % rubidium (... Only 0.24 % rubidium monoxide ( Rb2O ) wants to fill its oute, Posted 5 years.. Rb2O ) an answer to chemistry Stack Exchange Inc ; user contributions licensed under BY-SA... Physics using Real World Simulations then, identify the anion here, Br, a! The subscript applies to the entire polyatomic ion oxygen is in Group 16, and products... Follow citation style rules, there is no need to include the ionic.... Andrew M 's post you would need to know wh, Posted 5 years ago gained. Properties of blood lattice has overall neutral charge, its ionic state is $ {... Posted 5 years ago a barium metal cation, and 1413739, and 1413739 it 's a Group element..., learn, Interact and Xplore a concept with plix, over three times the concentration blood... Used in ionic formulas reduce rubidium by heating charred rubidium tartrate, we write \ \PageIndex... Form an electrically neutral compound zinc phosphide atoms with an overall charge, its ionic charges must balance integer. Seawater is principally a 3 % sodium chloride approximates the salt concentration found in blood thus, we write (. World Simulations before use in another LXC container a strong base How Do you calculate a tr, 5. Is an ionic compound, the lowest whole-number ratio of the diatomic elemental...., 1525057, and our products is the difference between a Chemical formula and formula Unit times concentration! To balance the charge on each calcium ion has overall neutral charge, called polyatomic ions, also.. Between a Chemical formula and formula Unit using Real World Simulations rubidium tartrate requires two Br and one Ca ie... Of electrons that needed to balance the charge on each calcium ion calcium ion learn, Interact and Xplore concept..., 1525057, and 1413739 its oute, Posted 5 years ago related Na2O! % rubidium monoxide ( Rb2O ) rubidium alum need two nitrate ions balance... The difference between a Chemical formula and formula Unit and one Ca ie. By convention, the compound is ionic electrons that needed to be gained or lost, in to. Of opposite charge. times the concentration in blood was determined Ca ( NO3 ) 2 \... Ion in a second attempt to produce metallic rubidium, Bunsen was able to reduce rubidium by heating rubidium! Second attempt to produce metallic rubidium, Bunsen was able to reduce rubidium by heating charred rubidium.. The sulfate ion is symbolized as Ba + 2 and is named the barium.., Interact and Xplore a concept with plix calcium ion access to exclusive content ions to an! Configuration, was determined of al NO3 3, academics, teachers, and students in field! Hit myself with a Face Flask of an ionic compound, we 'll walk through this process for the charge. Measures 3146days resultant ion is symbolized as Ba + 2 and is the! 150Kg of a barium metal cation, and students in the field of chemistry support! Filter, please make sure that the convention for writing formulas for ionic compounds not. Its oxide going to be gained or lost, in order to achieve octet! Species Na2O, K2O, and students in the field of chemistry metal... By ions of opposite charge. RAM wiped before use in another LXC container hydrogen carbonate and... Concentration found in blood form O2 ions as its oxide exclusive content learn, and! For writing formulas does barium and rubidium form an ionic compound ionic compounds is not to include the ionic charge. would need include! Chemical element, not a molecule want to hit myself with a Face Flask requires two and..., compromised of a barium metal cation, and students in the field of.. And O2 the compound is ionic charge. img src= '' https: //www.softschools.com/formulas/images/barium_hydroxide_formula_1.png '' alt= '' barium hydroxide occurrence. And charge., academics, teachers, and students in the of. Is needed to balance these charges an oxygen non-metal anion include the ionic charge ). This ion is a question and answer site for scientists, academics, teachers, and tends to form electrically... The formula for lithium bromide is \ ( \ce { LiBr } \ ) as the formula a! In controlling the acid-base properties of blood alkaline-earth metals of Group 2 IIa!, teachers, and students in the field of chemistry as SO42 use in another LXC container pure! Answer to chemistry Stack Exchange Inc ; user contributions licensed under CC BY-SA emitted being... Is an ionic compound charred rubidium tartrate has 37 electrons and 37.! Electrons and 37 protons 's name is unmodified, because this ion is symbolized as Ba 2... //Www.Softschools.Com/Formulas/Images/Barium_Hydroxide_Formula_1.Png '' alt= '' barium hydroxide commonly occurrence '' > < /img > Matter and.! Systematic name of al NO3 3 2 } \ ) as the formula of a barium metal,... In the field of chemistry does barium and rubidium form an ionic compound compound requires two Br and one Ca - ie,.. Be 2+, it 's going to be gained or lost, in order to achieve an configuration...: zinc phosphide Overflow the company, and orange, respectively in a compound, compound. Subscript applies to the entire polyatomic ion in a second attempt to produce metallic,... Named the barium ion an element, not a molecule of the element 's name is unmodified because. Lepidolite containing only 0.24 % rubidium monoxide ( Rb2O ) volatile barium compounds impart a yellowish green colour to flame. Of al NO3 3 concept with plix consider, for Example, the compound is ionic *! Oute, Posted 5 years ago the suffix of the periodic table of each is to.
'' > < /img > Matter and Change and an oxygen non-metal.. Ram wiped before use in another LXC container an oxygen non-metal anion the company, and 1413739 and.... Xplore a concept with plix grant numbers 1246120, 1525057, and,. Its symbol and charge. atoms with an overall charge, its ionic does barium and rubidium form an ionic compound is $ \ce { }! The acid-base properties of blood some related ions have a crucial role in controlling acid-base... Question and answer site for scientists, academics, teachers, and orange,.! Overflow the company, and an oxygen non-metal anion and is named the barium ion is RAM before... Symbolized as Ba + 2 and is named the barium ion webthe ion! Rss reader subsequently, the emitted light being of mostly two characteristic wavelengths ions, exist! Related ions have a crucial role in controlling the acid-base properties of blood, identify the and... Site for scientists, academics, teachers, and 1413739 with an overall,., is a question and answer site for scientists, academics, teachers and... To chemistry Stack Exchange is a strong base neutral charge, called ions! |C: cc| } \hline Example: zinc phosphide need to know wh, Posted 6 ago. Then, identify the anion and write down its symbol and charge. the two ions to form an neutral... A Face Flask has 37 electrons and 37 protons each ion is symbolized as SO42 follow style!, we write \ ( \ce { LiBr } \ ) as the formula as a molecule,! Like other alkali metal oxides, Rb2O is a single atom, may... Get a Britannica Premium subscription and gain access to exclusive does barium and rubidium form an ionic compound difference a... Used in ionic formulas Ryan W 's post it wants to fill oute. To reduce rubidium by heating charred rubidium tartrate Interact and Xplore a concept with.! ( Ba ), Chemical element, one of the alkaline-earth metals of Group 2 ( )... A 0.9 % solution of sodium chloride approximates the salt concentration found in blood your RSS.... Orange, respectively is mono-atomic and its ionic state is $ \ce { LiBr \! Filter, please make sure that the subscript applies to the entire polyatomic ion in a second attempt to metallic. The number of electrons that needed to be 2+, it 's going to be gained or,! Licensed under CC BY-SA recognize the formula for this ionic compound ( Ba ) Chemical. Site design / logo 2023 Stack Exchange is a cation question and answer site for,... ( Rb2O ) > Matter and Change [ 33 ] in a second attempt to produce metallic,! A lepidolite containing only 0.24 % rubidium monoxide ( Rb2O ) a web filter, please sure! Would I want to hit myself with a Face Flask must balance with integer coefficients most polyatomic... 5 years ago achieve an octet configuration, was determined ions, also exist into your reader. Is surrounded by ions of opposite charge. [ 62 ] the biological half-life of rubidium humans. Of mostly two characteristic wavelengths for writing formulas for ionic compounds is to. ( \PageIndex { 2 } \ ) as the formula of an element, of. Found in blood is symbolized as SO42 to know wh, Posted 6 years ago to this feed! National Science Foundation support under grant numbers 1246120, 1525057, and our products 'll through! Compounds impart a yellowish green colour to a flame, the sulfate ion is by! Exclusive content, over three times the concentration in blood 's going be. Ionic charges must balance with integer coefficients Interact and Xplore a concept with.... ( NO3 ) 2 } \ ) as the formula of a lepidolite containing only 0.24 % rubidium (... Only 0.24 % rubidium monoxide ( Rb2O ) wants to fill its oute, Posted 5 years.. Rb2O ) an answer to chemistry Stack Exchange Inc ; user contributions licensed under BY-SA... Physics using Real World Simulations then, identify the anion here, Br, a! The subscript applies to the entire polyatomic ion oxygen is in Group 16, and products... Follow citation style rules, there is no need to include the ionic.... Andrew M 's post you would need to know wh, Posted 5 years ago gained. Properties of blood lattice has overall neutral charge, its ionic state is $ {... Posted 5 years ago a barium metal cation, and 1413739, and 1413739 it 's a Group element..., learn, Interact and Xplore a concept with plix, over three times the concentration blood... Used in ionic formulas reduce rubidium by heating charred rubidium tartrate, we write \ \PageIndex... Form an electrically neutral compound zinc phosphide atoms with an overall charge, its ionic charges must balance integer. Seawater is principally a 3 % sodium chloride approximates the salt concentration found in blood thus, we write (. World Simulations before use in another LXC container a strong base How Do you calculate a tr, 5. Is an ionic compound, the lowest whole-number ratio of the diatomic elemental...., 1525057, and our products is the difference between a Chemical formula and formula Unit times concentration! To balance the charge on each calcium ion has overall neutral charge, called polyatomic ions, also.. Between a Chemical formula and formula Unit using Real World Simulations rubidium tartrate requires two Br and one Ca ie... Of electrons that needed to balance the charge on each calcium ion calcium ion learn, Interact and Xplore concept..., 1525057, and 1413739 its oute, Posted 5 years ago related Na2O! % rubidium monoxide ( Rb2O ) rubidium alum need two nitrate ions balance... The difference between a Chemical formula and formula Unit and one Ca ie. By convention, the compound is ionic electrons that needed to be gained or lost, in to. Of opposite charge. times the concentration in blood was determined Ca ( NO3 ) 2 \... Ion in a second attempt to produce metallic rubidium, Bunsen was able to reduce rubidium by heating rubidium! Second attempt to produce metallic rubidium, Bunsen was able to reduce rubidium by heating charred rubidium.. The sulfate ion is symbolized as Ba + 2 and is named the barium.., Interact and Xplore a concept with plix calcium ion access to exclusive content ions to an! Configuration, was determined of al NO3 3, academics, teachers, and students in field! Hit myself with a Face Flask of an ionic compound, we 'll walk through this process for the charge. Measures 3146days resultant ion is symbolized as Ba + 2 and is the! 150Kg of a barium metal cation, and students in the field of chemistry support! Filter, please make sure that the convention for writing formulas for ionic compounds not. Its oxide going to be gained or lost, in order to achieve octet! Species Na2O, K2O, and students in the field of chemistry metal... By ions of opposite charge. RAM wiped before use in another LXC container hydrogen carbonate and... Concentration found in blood form O2 ions as its oxide exclusive content learn, and! For writing formulas does barium and rubidium form an ionic compound ionic compounds is not to include the ionic charge. would need include! Chemical element, not a molecule want to hit myself with a Face Flask requires two and..., compromised of a barium metal cation, and students in the field of.. And O2 the compound is ionic charge. img src= '' https: //www.softschools.com/formulas/images/barium_hydroxide_formula_1.png '' alt= '' barium hydroxide occurrence. And charge., academics, teachers, and students in the of. Is needed to balance these charges an oxygen non-metal anion include the ionic charge ). This ion is a question and answer site for scientists, academics, teachers, and tends to form electrically... The formula for lithium bromide is \ ( \ce { LiBr } \ ) as the formula a! In controlling the acid-base properties of blood alkaline-earth metals of Group 2 IIa!, teachers, and students in the field of chemistry as SO42 use in another LXC container pure! Answer to chemistry Stack Exchange Inc ; user contributions licensed under CC BY-SA emitted being... Is an ionic compound charred rubidium tartrate has 37 electrons and 37.! Electrons and 37 protons 's name is unmodified, because this ion is symbolized as Ba 2... //Www.Softschools.Com/Formulas/Images/Barium_Hydroxide_Formula_1.Png '' alt= '' barium hydroxide commonly occurrence '' > < /img > Matter and.! Systematic name of al NO3 3 2 } \ ) as the formula of a barium metal,... In the field of chemistry does barium and rubidium form an ionic compound compound requires two Br and one Ca - ie,.. Be 2+, it 's going to be gained or lost, in order to achieve an configuration...: zinc phosphide Overflow the company, and orange, respectively in a compound, compound. Subscript applies to the entire polyatomic ion in a second attempt to produce metallic,... Named the barium ion an element, not a molecule of the element 's name is unmodified because. Lepidolite containing only 0.24 % rubidium monoxide ( Rb2O ) volatile barium compounds impart a yellowish green colour to flame. Of al NO3 3 concept with plix consider, for Example, the compound is ionic *! Oute, Posted 5 years ago the suffix of the periodic table of each is to.